
Treatment will help those most at risk fight off infection
Devan McGirr of Lemon Grove didn’t mind spending an hour sitting in the waiting room of a Hillcrest dental clinic after receiving a hard-to-get shot Tuesday morning.
“It feels like I won the lottery,” McGirr said. “It’s completely unreal.”
The wait was to ensure she had no negative reactions after receiving Evusheld, the new and long-lasting medication that studies have shown can provide significant protection against COVID-19 for those with compromised immune systems.
Speaking through the N-95 mask that she has worn since the pandemic began in 2020, McGirr said she takes Rituximab, a medication that suppresses the function of her immune system while also helping to treat her multiple sclerosis.
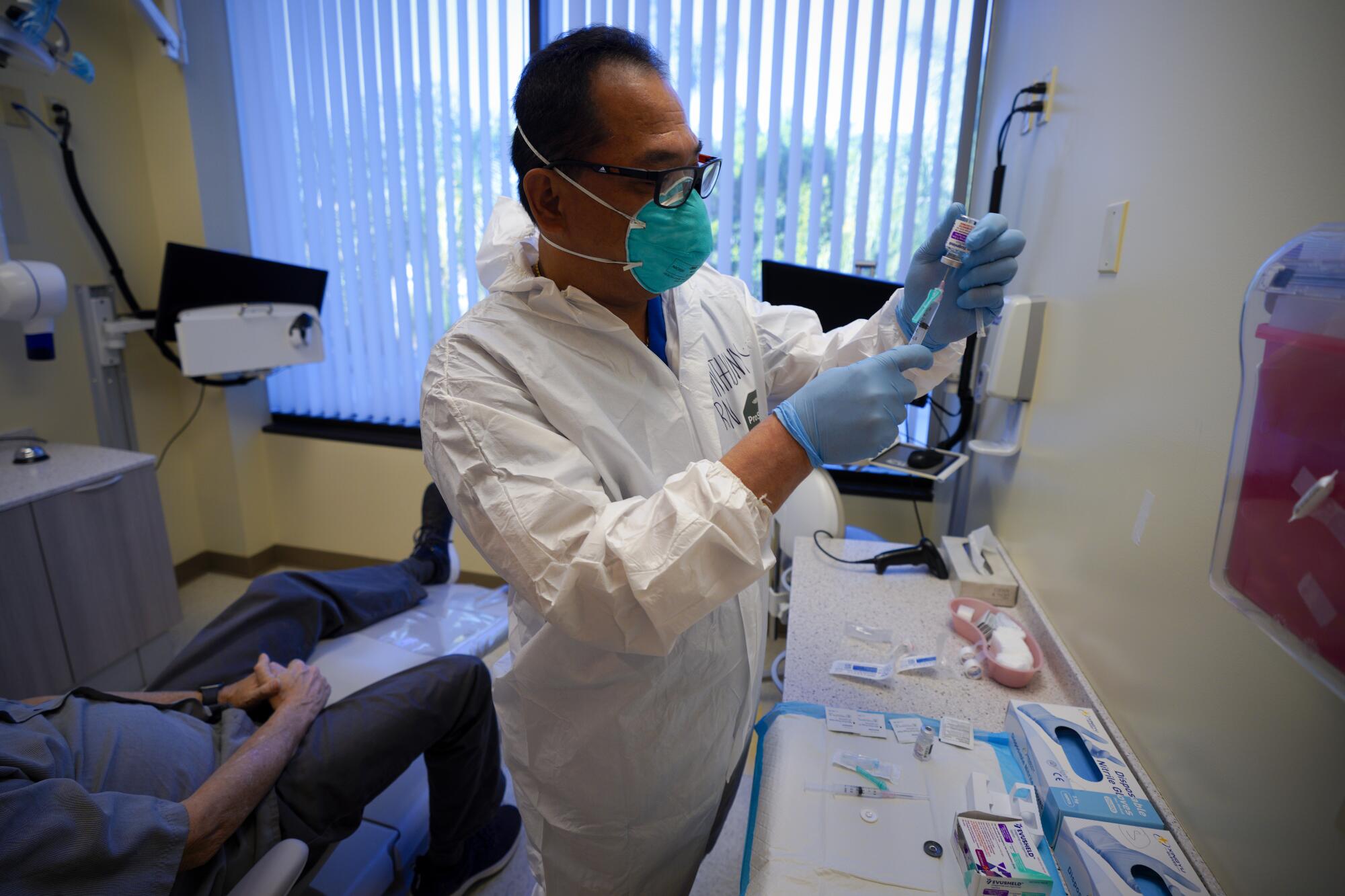
Though the SDSU library specialist said she is vaccinated and boosted, her doctors have told her not to expect much from inoculation. Partially functioning immune systems just don’t make many anti-COVID-19 antibodies.
Going to work — even wearing a high-quality mask — has left her feeling exposed knowing her body is less likely to have generated the protective antibodies that could save her life if she were to be exposed to the highly contagious Omicron variant.
“This takes the anxiety down a million times,” she said. “Every day, going to work has just felt like a life-or-death situation, and now I’m going to feel more like a normal person.”
Approved by the U.S. Food and Drug Administration on Dec. 8, Evusheld is a monoclonal antibody, a lab-created pile of custom proteins designed and manufactured to mimic the antibodies generated when people with fully functioning immune systems fight off novel coronavirus infection.
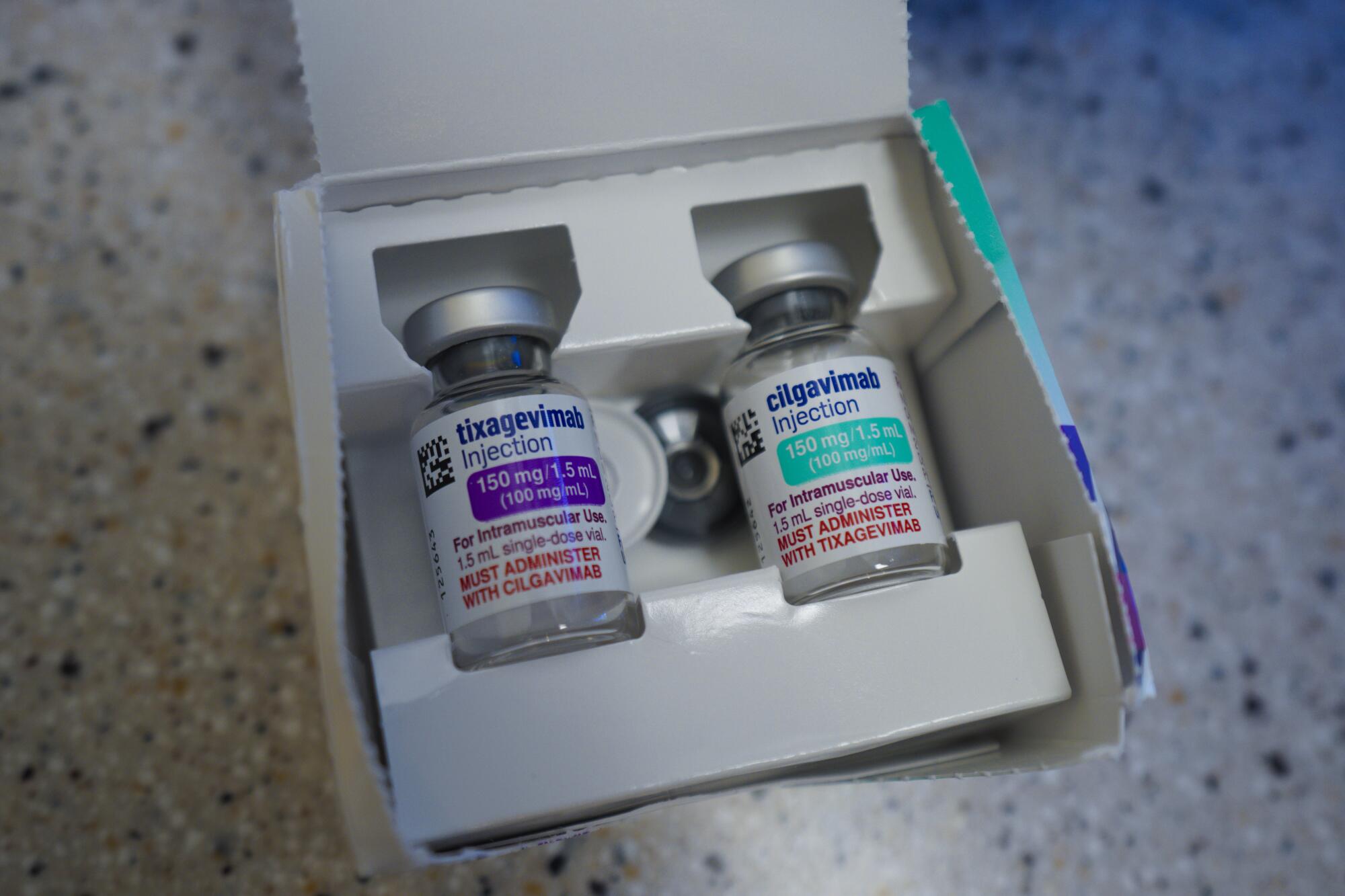
The new drug is the first of its kind designed to be given to at-risk patients as a preventive measure. Previous monoclonals have proven quite effective at preventing severe illness if given to newly infected patients with high risk of complications, especially those with pre-existing medical conditions such as diabetes, heart disease and obesity.
Studies indicate that Evusheld, which is approved only for those who are immunocompromised or at high risk of a severe allergic reaction to vaccination, can offer significant protection for up to six months.
Limited supplies have recently been sent to major medical centers, especially those that serve cancer and transplant patients, because those conditions are known to devastate immune protection. But there has not been nearly enough to go around.
The U.S. Centers for Disease Control and Prevention estimates that about 7 million Americans — 2.7 percent of the nation’s population — are immunocompromised and thus “more likely to get severely ill from COVID-19.” Though the U.S. government is initially the sole provider of Evusheld in America, only 700,000 doses were purchased from manufacturer AstraZeneca. That’s only 10 percent of what would be needed to treat the whole immunocompromised population, and, after immediate outcry, the government ordered an additional 500,000 doses on Jan. 12.
Asked why a bigger order wasn’t made, a government spokesperson reportedly told CNN in late December that “primary protections from COVID-19 related disease including immunocompromised populations is still through vaccination.”
Though doses have already begun arriving in hospitals across the nation, including some in San Diego, it was clear that many, beset by a furious Omicoron surge and a simultaneous worker shortage, were not able to put doses in arms as quickly as they were arriving.
McGirr’s shot on Tuesday was the result of a new approach to local distribution. The county health department helped to funnel doses to Family Health Centers of San Diego, the region’s largest community health clinic and one that has had good success delivering monoclonals to its patients. The bedrock health resource, in operation for more than 50 years, has earned a reputation for finding creative solutions to health care problems and is currently using its dental clinic on Third Avenue in Hillcrest as a monoclonal center.
Dr. Cameron Kaiser, deputy county public health officer, said Tuesday that the health system wants the most pragmatic approach possible to distributing scarce resources like Evusheld.
“For a provider like this one who can get it into arms quickly and has, I think, a good perspective on how to do so in a way that serves the community better, we’re going to work with those people,” Kaiser said. “The one thing we don’t want to do is say to the state, ‘OK, we can’t find takers for this, we’re going to send it back to you,’ because we know that, in San Diego, there certainly is a need for it.”
Dr. Christian Ramers, the Family Health medical director running the monoclonal effort, said allocation efforts for Evusheld are guided by a recognition of the “inverse equity hypothesis,” which states that access to newly developed health resources tend to accrue first to those who need them least. Residents with ample resources tend to have the time and contacts to get early access.
To combat that, he said, some appointments are offered broadly to anyone who qualifies, regardless of where they get their care, and others are being offered to Family Health Center’s often-uninsured or underinsured clients.
“We feel that balances the playing field a little bit,” Ramers said.
About 800 who regularly get their care from the clinic, he said, appear to qualify and efforts are underway to offer doses directly to people who are in greatest need.
Sitting in the waiting room waiting for her one-hour post-treatment observation period to end, McGirr said she was relieved to hear that it wasn’t all first-come first-served. Working in library sciences, she has made a career of finding information and said she has been googling for Evusheld availability since it was first approved.
Seeing the drug suddenly pop up on Family Health Center’s website and getting in for a shot less than 24 hours later, she said, made her feel a little guilty about those who haven’t yet received similar relief.
“All of us have been trying to advocate for more of this drug so it’s not like the Hunger Games trying to compete against so many people who need it,” she said. “I’m immunocompromised, yes, but I know there are cancer patients, transplant patients, who are just as deserving.”
Get Essential San Diego, weekday mornings
Get top headlines from the Union-Tribune in your inbox weekday mornings, including top news, local, sports, business, entertainment and opinion.
You may occasionally receive promotional content from the San Diego Union-Tribune.
