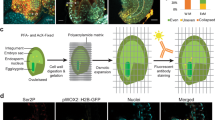
Abstract
After double fertilization, the endosperm in the seeds of many flowering plants undergoes repeated mitotic nuclear divisions without cytokinesis, resulting in a large coenocytic endosperm that then cellularizes. Growth during the coenocytic phase is strongly associated with the final seed size; however, a detailed description of the cellular dynamics controlling the unique coenocytic development in flowering plants has remained elusive. By integrating confocal microscopy live-cell imaging and genetics, we have characterized the entire development of the coenocytic endosperm of Arabidopsis thaliana including nuclear divisions, their timing intervals, nuclear movement and cytoskeleton dynamics. Around each nucleus, microtubules organize into aster-shaped structures that drive actin filament (F-actin) organization. Microtubules promote nuclear movement after division, while F-actin restricts it. F-actin is also involved in controlling the size of both the coenocytic endosperm and the mature seed. The characterization of cytoskeleton dynamics in real time throughout the entire coenocyte endosperm period provides foundational knowledge of plant coenocytic development, insights into the coordination of F-actin and microtubules in nuclear dynamics, and new opportunities to increase seed size and our food security.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
All data to support the conclusions of this manuscript are provided in the main figures and the extended data figures. Source data are provided with this paper.
References
Sharma, V., Clark, A. J. & Kawashima, T. Insights into the molecular evolution of fertilization mechanism in land plants. Plant Reprod. 34, 353–364 (2021).
Kawashima, T. & Berger, F. Green love talks: cell–cell communication during double fertilization in flowering plants. AoB PLANTS 2011, plr015 (2011).
Berger, F. Endosperm development. Curr. Opin. Plant Biol. 2, 28–32 (1999).
Brown, R. C., Lemmon, B. E., Nguyen, H. & Olsen, O.-A. Development of endosperm in Arabidopsis thaliana. Sex. Plant Reprod. 12, 32–42 (1999).
Olsen, O.-A. Nuclear endosperm development in cereals and Arabidopsis thaliana. Plant Cell 16, S214–S227 (2004).
Olsen, O.-A., Linnestad, C. & Nichols, S. E. Developmental biology of the cereal endosperm. Trends Plant Sci. 4, 253–257 (1999).
Brown, R. C., Lemmon, B. E. & Olsen, O.-A. Development of the endosperm in rice (Oryza sativa L.): cellularization. J. Plant Res. 109, 301–313 (1996).
Orozco-Arroyo, G., Paolo, D., Ezquer, I. & Colombo, L. Networks controlling seed size in Arabidopsis. Plant Reprod. 28, 17–32 (2015).
Boisnard-Lorig, C. et al. Dynamic analyses of the expression of the HISTONE::YFP fusion protein in Arabidopsis show that syncytial endosperm is divided in mitotic domains. Plant Cell 13, 495–509 (2001).
Kang, I.-H., Steffen, J. G., Portereiko, M. F., Lloyd, A. & Drews, G. N. The AGL62 MADS domain protein regulates cellularization during endosperm development in Arabidopsis. Plant Cell 20, 635–647 (2008).
Ingouff, M., Haseloff, J. & Berger, F. Polycomb group genes control developmental timing of endosperm. Plant J. 42, 663–674 (2005).
Ohad, N. et al. A mutation that allows endosperm development without fertilization. Proc. Natl Acad. Sci. USA 93, 5319–5324 (1996).
Scott, R. J., Spielman, M., Bailey, J. & Dickinson, H. G. Parent-of-origin effects on seed development in Arabidopsis thaliana. Development 125, 3329–3341 (1998).
Lafon-Placette, C. et al. Endosperm-based hybridization barriers explain the pattern of gene flow between Arabidopsis lyrata and Arabidopsis arenosa in Central Europe. Proc. Natl Acad. Sci. USA 114, E1027–E1035 (2017).
Ohto, M., Floyd, S. K., Fischer, R. L., Goldberg, R. B. & Harada, J. J. Effects of APETALA2 on embryo, endosperm, and seed coat development determine seed size in Arabidopsis. Sex. Plant Reprod. 22, 277–289 (2009).
Garcia, D. et al. Arabidopsis haiku mutants reveal new controls of seed size by endosperm. Plant Physiol. 131, 1661–1670 (2003).
Zhang, B., Li, C., Li, Y. & Yu, H. Mobile TERMINAL FLOWER1 determines seed size in Arabidopsis. Nat. Plants 6, 1146–1157 (2020).
Ali, M. F. et al. ARP2/3-independent WAVE/SCAR pathway and class XI myosin control sperm nuclear migration in flowering plants. Proc. Natl Acad. Sci. USA 117, 32757–32763 (2020).
Kawashima, T. et al. Dynamic F-actin movement is essential for fertilization in Arabidopsis thaliana. eLife 3, e04501 (2014).
Kimata, Y. et al. Cytoskeleton dynamics control the first asymmetric cell division in Arabidopsis zygote. Proc. Natl Acad. Sci. USA 113, 14157–14162 (2016).
Brown, R. C., Lemmon, B. E. & Nguyen, H. Events during the first four rounds of mitosis establish three developmental domains in the syncytial endosperm of Arabidopsis thaliana. Protoplasma 222, 167–174 (2003).
Brown, R. C. & Lemmon, B. E. The cytoskeleton and spatial control of cytokinesis in the plant life cycle. Protoplasma 215, 35–49 (2001).
Nguyen, H., Brown, R. C. & Lemmon, B. E. Patterns of cytoskeletal organization reflect distinct developmental domains in endosperm of Coronopus didymus (Brassicaceae). Int. J. Plant Sci. 162, 1–14 (2001).
Brown, R. C., Lemmon, B. E. & Olsen, O. A. Endosperm development in barley: microtubule involvement in the morphogenetic pathway. Plant Cell 6, 1241–1252 (1994).
González-Gutiérrez, A. G., Gutiérrez-Mora, A., Verdín, J. & Rodríguez-Garay, B. An F-actin mega-cable is associated with the migration of the sperm nucleus during the fertilization of the polarity-inverted central cell of Agave inaequidens. Front. Plant Sci. 12, 774098 (2021).
Ali, M. F. & Kawashima, T. Formins control dynamics of F-actin in the central cell of Arabidopsis thaliana. Plant Signal. Behav. 16, 1920192 (2021).
Fatema, U., Ali, M. F., Hu, Z., Clark, A. J. & Kawashima, T. Gamete nuclear migration in animals and plants. Front. Plant Sci. 10, 517 (2019).
Shin, J. M., Yuan, L., Ohme-Takagi, M. & Kawashima, T. Cellular dynamics of double fertilization and early embryogenesis in flowering plants. J. Exp. Zool. B https://doi.org/10.1002/jez.b.22981 (2020).
Schmit, A. C. & Lambert, A. M. Characterization and dynamics of cytoplasmic F-actin in higher plant endosperm cells during interphase, mitosis, and cytokinesis. J. Cell Biol. 105, 2157–2166 (1987).
Kinoshita, T. et al. One-way control of FWA imprinting in Arabidopsis endosperm by DNA methylation. Science 303, 521–523 (2004).
Faure, J.-E., Rotman, N., Fortuné, P. & Dumas, C. Fertilization in Arabidopsis thaliana wild type: developmental stages and time course. Plant J. 30, 481–488 (2002).
Maruyama, D., Higashiyama, T., Endo, T. & Nishikawa, S.-I. Fertilization-coupled sperm nuclear fusion is required for normal endosperm nuclear proliferation. Plant Cell Physiol. https://doi.org/10.1093/pcp/pcz158 (2019).
Kato, T., Morita, M. T. & Tasaka, M. Defects in dynamics and functions of actin filament in Arabidopsis caused by the dominant-negative actin fiz1-induced fragmentation of actin filament. Plant Cell Physiol. 51, 333–338 (2010).
Belyy, A., Merino, F., Sitsel, O. & Raunser, S. Structure of the Lifeact–F-actin complex. PLoS Biol. 18, e3000925 (2020).
Kumari, A., Kesarwani, S., Javoor, M. G., Vinothkumar, K. R. & Sirajuddin, M. Structural insights into actin filament recognition by commonly used cellular actin markers. EMBO J. 39, e104006 (2020).
Picard, C. L., Povilus, R. A., Williams, B. P. & Gehring, M. Transcriptional and imprinting complexity in Arabidopsis seeds at single-nucleus resolution. Nat. Plants 7, 730–738 (2021).
Belmonte, M. F. et al. Comprehensive developmental profiles of gene activity in regions and subregions of the Arabidopsis seed. Proc. Natl Acad. Sci. USA 110, E435–E444 (2013).
Kawashima, T. & Berger, F. The central cell nuclear position at the micropylar end is maintained by the balance of F-actin dynamics, but dispensable for karyogamy in Arabidopsis. Plant Reprod. 28, 103–110 (2015).
Suarez, C. et al. Profilin regulates F-actin network homeostasis by favoring formin over Arp2/3 complex. Dev. Cell 32, 43–53 (2015).
Kandasamy, M. K., McKinney, E. C. & Meagher, R. B. Functional nonequivalency of actin isovariants in Arabidopsis. Mol. Biol. Cell 13, 251–261 (2002).
Canaday, J. et al. Microtubule assembly in higher plants. Recent Res. Dev. Mol. Biol. 2, 103–119 (2004).
Masoud, K., Herzog, E., Chabouté, M.-E. & Schmit, A.-C. Microtubule nucleation and establishment of the mitotic spindle in vascular plant cells. Plant J. Cell Mol. Biol. 75, 245–257 (2013).
Morejohn, L. C., Bureau, T. E., Molè-Bajer, J., Bajer, A. S. & Fosket, D. E. Oryzalin, a dinitroaniline herbicide, binds to plant tubulin and inhibits microtubule polymerization in vitro. Planta 172, 252–264 (1987).
Cui, Y., Zhao, Q., Hu, S. & Jiang, L. Vacuole biogenesis in plants: how many vacuoles, how many models? Trends Plant Sci. 25, 538–548 (2020).
Marty, F. Plant vacuoles. Plant Cell 11, 587–600 (1999).
Cui, Y. et al. A whole-cell electron tomography model of vacuole biogenesis in Arabidopsis root cells. Nat. Plants 5, 95–105 (2019).
Zheng, W. et al. AP3M harbors actin filament binding activity that is crucial for vacuole morphology and stomatal closure in Arabidopsis. Proc. Natl Acad. Sci. USA 116, 18132–18141 (2019).
Scheuring, D. et al. Actin-dependent vacuolar occupancy of the cell determines auxin-induced growth repression. Proc. Natl Acad. Sci. USA 113, 452–457 (2016).
Uemura, T., Yoshimura, S. H., Takeyasu, K. & Sato, M. H. Vacuolar membrane dynamics revealed by GFP–AtVam3 fusion protein. Genes Cells Devoted Mol. Cell. Mech. 7, 743–753 (2002).
Lv, Z. et al. The emergent yo-yo movement of nuclei driven by cytoskeletal remodeling in pseudo-synchronous mitotic cycles. Curr. Biol. 30, 2564–2573.e5 (2020).
Lv, Z., de-Carvalho, J., Telley, I. A. & Großhans, J. Cytoskeletal mechanics and dynamics in the Drosophila syncytial embryo. J. Cell Sci. 134, jcs246496 (2021).
Sullivan, W. & Theurkauf, W. E. The cytoskeleton and morphogenesis of the early Drosophila embryo. Curr. Opin. Cell Biol. 7, 18–22 (1995).
Karr, T. L. & Alberts, B. M. Organization of the cytoskeleton in early Drosophila embryos. J. Cell Biol. 102, 1494–1509 (1986).
Sommi, P., Cheerambathur, D., Brust-Mascher, I. & Mogilner, A. Actomyosin-dependent cortical dynamics contributes to the prophase force-balance in the early Drosophila embryo. PLoS ONE 6, e18366 (2011).
Ingouff, M. et al. Plant formin AtFH5 is an evolutionarily conserved actin nucleator involved in cytokinesis. Nat. Cell Biol. 7, 374–380 (2005).
Nguyen, H., Brown, R. C. & Lemmon, B. E. The specialized chalazal endosperm in Arabidopsis thaliana and Lepidium virginicum (Brassicaceae). Protoplasma 212, 99–110 (2000).
Baroux, C., Fransz, P. & Grossniklaus, U. Nuclear fusions contribute to polyploidization of the gigantic nuclei in the chalazal endosperm of Arabidopsis. Planta 220, 38–46 (2004).
Fitz Gerald, J. N., Hui, P. S. & Berger, F. Polycomb group-dependent imprinting of the actin regulator AtFH5 regulates morphogenesis in Arabidopsis thaliana. Dev. Camb. Engl. 136, 3399–3404 (2009).
Qiu, J.-L., Jilk, R., Marks, M. D. & Szymanski, D. B. The Arabidopsis SPIKE1 gene is required for normal cell shape control and tissue development. Plant Cell 14, 101–118 (2002).
Smith, L. G. Cytoskeletal control of plant cell shape: getting the fine points. Curr. Opin. Plant Biol. 6, 63–73 (2003).
Beauzamy, L. et al. Endosperm turgor pressure decreases during early Arabidopsis seed development. Development 143, 3295–3299 (2016).
Sheahan, M. B., Rose, R. J. & McCurdy, D. W. Actin-filament-dependent remodeling of the vacuole in cultured mesophyll protoplasts. Protoplasma 230, 141–152 (2007).
Clough, S. J. & Bent, A. F. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. Cell Mol. Biol. 16, 735–743 (1998).
Gooh, K. et al. Live-cell imaging and optical manipulation of Arabidopsis early embryogenesis. Dev. Cell 34, 242–251 (2015).
Braselton, J. P., Wilkinson, M. J. & Clulow, S. A. Feulgen staining of intact plant tissues for confocal microscopy. Biotech. Histochem. 71, 84–87 (1996).
Herridge, R. P., Day, R. C., Baldwin, S. & Macknight, R. C. Rapid analysis of seed size in Arabidopsis for mutant and QTL discovery. Plant Methods 7, 3 (2011).
Acknowledgements
We thank A. Clark and D. Zaitlin for their critical comments on this manuscript and Y. Ohnishi for the endosperm area analysis. This work was supported by NSF grant no. IOS-1928836 (to T.K.), JSPS KAKENHI JP22H04668 (to D.K.) and JST FOREST Program JPMJFR204T (to D.K.).
Author information
Authors and Affiliations
Contributions
M.F.A., J.M.S. and T.K. conceived and designed the experiments. M.F.A., J.M.S., U.F., D.K. and T.K. performed the experiments. M.F.A., J.M.S., U.F., D.K., F.B. and T.K. contributed the materials and analysis tools. M.F.A., J.M.S., F.B., L.Y. and T.K. analysed the data. M.F.A., J.M.S., F.B., L.Y. and T.K. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Plants thanks Gwyneth Ingram, Enrico Magnani and Chen Chen for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 F-actin does not affect endosperm cellularization.
Z-projected confocal images of Feulgen-stained endosperm at 5−6 d after pollination (DAP). Control (a−b), OX-ACTIN (c−d), and DN-ACTIN (e−f). Endosperm cellularization was already observed in the control and OX-ACTIN at 5 DAP, whereas no endosperm cellularization had initiated at the time in DN-ACTIN. The experiments in a−f were repeated two times independently with similar results. Scale bar, 20 µm.
Extended Data Fig. 2 F-actin and MT aster structures become more apparent after the 3rd nuclear divisions.
a, Illustration showing how to set the axis for analyzing frequency histograms of spatial orientations of F-actin and MTs surrounding the endosperm nucleus (related to Figs. 2c−e and 4a−c) and definition of the criteria to evaluate aster structure. Magenta, nucleus; cyan, F-actin or MTs. b,c, Probability distribution of F-actin (b) and MTs (c) in degrees relative to the axis of a quarter nucleus (bottom in a). The probability is calculated from all frequency histograms of the orientations of F-actin and MTs combined from the entire nucleus (top in a). Different colors denote the different nuclear division stages. The shaded area indicates reticulate patterns of F-actin and MTs, which do not contribute to the aster structures.
Extended Data Fig. 3 The displacement of daughter nuclei after mitotic nuclear division.
a, Z-projected confocal image of coenocytic endosperm nuclei (marked by different colored ovals) and their movement 2 h after division. Nuclear trajectories of daughter nuclei after division are shown in magenta. Scale bar 20 µm. b, Average nuclear displacement between the 5th and 6th nuclear divisions. The dots on the bar plot represent the individual data points; 5th division, n = 4 seeds (total 17 nuclei); 6th division, n = 5 seeds (total 20 nuclei). Error bars represent the standard error (SE). Data are presented as the mean + SE on the bar plot. Levels not connected by the same letter on the graph b are significantly different at p < 0.01. An unpaired t-test analysis was applied, and the P value (two-tailed) was 0.639.
Extended Data Fig. 4 Independent transgenic lines show consistent phenotypes.
a−b, Quantitative analysis of actin bundles around each nucleus (a) and internuclear distance (b) in coenocytic endosperm after the 5th division of additional transgenic lines. Individual dots represent the means of actin bundle number and internuclear distance per seed, and the middle bars on the dot plots represent the mean of the means. The control, n = 10 seeds; OX-ACTIN-#2, n = 9 seeds in a. The control, n = 12 seeds; DN-ACTIN-#2, n = 14 seeds, OX-ACTIN-#2, n = 14 seeds in b. Error bars represent the standard errors. NA in a denotes not applicable, because of no actin bundles. c, Quantitative analysis of seed size of mature seeds. The seed size of each line is represented by 900 seeds per plant from four individual plants (n = 3,600 seeds). The middle black dotted and solid lines within the plot show the median with interquartile range. Levels not connected by the same letter (a–c) are significantly different at p < 0.01. In a, an unpaired t-test analysis was applied, and the P value (two-tailed) was 4E-06. In b, one-way ANOVA, Tukey-Kramer HSD test was applied, and the P values for the control versus DN-ACTIN-#2, the control versus OX-ACTIN-#2 were 0.002, and 0.0006, respectively. In c, Games-Howell test was applied, and the P values for the control versus DN-ACTIN-#2, the control versus OX-ACTIN-#2, were 6.24E-12 and 1.06E-11, respectively.
Extended Data Fig. 5 F-actin does not control nuclear division and intervals.
Coenocytic endosperm nuclear division numbers and intervals of the control (top), OX-ACTIN (middle) and DN-ACTIN (bottom). Horizontal arrow bars represent the duration from pollination until endosperm cellularization and the duration represents the minimum and maximum of the total nuclear division intervals of individual samples in each line. Horizontal lines with vertical bars represent the average length of each nuclear division interval. Distances between vertical bars are drawn to scale. In DN-ACTIN, we could not observe the 9th and 10th division intervals due to the death of the samples under long time-lapse live-cell imaging conditions; however, the total duration was estimated based on the cellularization timing analyzed by Feulgen staining analysis (Fig. 6d). ND, not determined.
Extended Data Fig. 6 Disorganized reticulate F-actin at the moment when the MTs form spindles during mitotic nuclear division.
a−f, Z-projected confocal images of coenocytic endosperm F-actin (a–d, cyan, proFWA::Lifeact-Venus), MTs (b–e, yellow, proFWA::TagRFP-TUA5) and the two merged (c–f) before and/or after division (a−c) and at the onset of mitotic nuclear division (d−f). The experiments in a−f were repeated two times independently with similar results. Scale bar, 20 µm. The arrowhead indicates MT spindle formation during mitotic nuclear division and the arrow indicates disorganized reticulate F-actin in f.
Extended Data Fig. 7 Dose-dependent effect of oryzalin in the inhibition of MT and F-actin aster structures.
a−r, Snapshots and time-lapse Z-projected confocal images of the semi-in vivo cultured coenocytic endosperm MT (yellow, proFWA::TagRFP-TUA5), F-actin (cyan, proFWA::Lifeact-Venus), and nuclei (magenta, proFWA::H2B-mRuby2). MT control (a−c), MTs at 10 µM oryzalin (d−f), MT at 20 µM oryzalin (g−i), F-actin control (j−l), F-actin at 10 µM oryzalin (m−o), F-actin at 20 µM oryzalin (p−q). Enlarged inserts at the bottom right of each image represent the F-actin and MTs around the nucleus. The experiments in a−r were repeated two times independently with similar results. Scale bar 20 µm.
Extended Data Fig. 8 F-actin is not required for MT function during coenocytic endosperm development.
a−d, Time-lapse Z-projected confocal images of the semi-in vivo cultured coenocytic endosperm MT (yellow, proFWA::TagRFP-TUA5) in the control (a–b), and 5 μM Lat B treatment (c–d). Lat B treatment did not affect MT organization (c) and nuclear division (d) during coenocytic endosperm development. Arrowheads indicate MT aster radiating from each nucleus and arrows indicate MT spindles formed during mitotic nuclear division. The experiments in a−d were repeated two times independently with similar results. Scale bar, 20 µm.
Extended Data Fig. 9 Growth similarity between in planta and semi-in vivo cultured seeds as well as in the vegetative phase among the lines.
a, Average area of the coenocytic endosperm in the semi-in vivo cultured control, DN-ACTIN, and OX-ACTIN seeds from 3 to 5 DAP. The dots on the bar plot represent the individual data points (the control seeds at 3–5 DAP, n = 11, 10 and 8, respectively; DN-ACTIN seeds at 3–5 DAP, n = 11, 9 and 5, respectively; OX-ACTIN seeds at 3–5 DAP, n = 8, 8 and 5, respectively). Error bars represent the standard deviation (SD). Data are presented as the mean + SD on the bar plot. Endosperm areas at the same stage were compared statistically. Levels not connected by the same letter (a–c) are significantly different (p < 0.1, one-way ANOVA, Tukey-Kramer HSD test). At 3 DAP, the P values for the control versus DN-ACTIN and OX-ACTIN were 0.5567 and 0.0118, respectively. At 4 DAP, the P values for the control versus DN-ACTIN and OX-ACTIN were 0.09 and 0.0069, respectively. At 5 DAP, the P values for the control versus DN-ACTIN and OX-ACTIN were 0.07 and 0.02, respectively. b−c, No significant differences in plant growth and development; leaf structure (b), plant height (c) in DN-ACTIN and OX-ACTIN compared to the control.
Extended Data Fig. 10 The size of the endosperm cell correlates with the size of the coenocytic endosperm.
a−f, Confocal images of Feulgen-stained cellularized endosperm of the control (a−b), DN-ACTIN (c−d), and OX-ACTIN (e−f) at 6 d after pollination (DAP). Red marks indicate the area of the cellularized endosperm cell. The experiments in a−f were repeated two times independently with similar results. Scale bar 50 µm. a-f correspond to Supplementary Video 11. g, Quantitative analysis of the area of the cellularized endosperm cell. Individual dots represent the means of cell size per seed, and the middle bars on the dot plots represent the mean of the means. Error bars represent the standard errors. 15–20 cells per seed were analyzed. The control, n = 7 seeds; DN-ACTIN, n = 5 seeds; OX-ACTIN, n = 5 seeds. Levels connected by the same letter (a) are significantly not different (p < 0.05, one-way ANOVA, Tukey-Kramer HSD test). The P values for the control versus DN-ACTIN and the control versus OX-ACTIN were 0.954 and 0.157, respectively.
Supplementary information
Supplementary Information
Supplementary Tables 1 and 2.
Supplementary Video 1
Combined time-lapse live-cell video showing the 1st to 10th nuclear divisions in the Arabidopsis coenocytic endosperm. Endosperm nuclei are marked by proFWA::H2B–mRuby2 (magenta) (related to Fig. 1). The first seed shows the 1st to 4th divisions (20 min intervals, 18 h in total), the 2nd seed shows the 4th to 16th divisions (15 min intervals, 29 h in total), the 3rd seed shows the 6th to 7th divisions (20 min intervals, 29 h in total) and the 4th seed shows the 8th to 10th divisions (20 min intervals, 44 h in total). Note that the cable structures are also visible in the 2nd and 4th seeds due to the simultaneous excitation of Lifeact–Venus (proFWA::Lifeact–Venus) by a 514 nm laser. The unspecific signal detected in the RFP channel in seed coat layers and dissected tissue debris is autofluorescence. Scale bar, 20 µm.
Supplementary Video 2
3D structure of endosperm showing F-actin and endosperm nuclei visualized by proFWA::Lifeact–Venus (cyan) and proFWA::H2B–mRuby2 (magenta), respectively, in Arabidopsis coenocytic endosperm (related to Fig. 2).
Supplementary Video 3
Combined time-lapse live-cell video of F-actin dynamics and endosperm nuclei visualized by proFWA::Lifeact–Venus (cyan) and proFWA::H2B–mRuby2 (magenta), respectively, in Arabidopsis coenocytic endosperm (related to Fig. 2). The 1st seed shows the early stage of coenocytic endosperm (15 min intervals, 16 h in total), and the 2nd seed shows the late stage of coenocytic endosperm (20 min intervals, 27 h in total). The unspecific signal detected in the RFP channel in seed coat layers and dissected tissue debris is autofluorescence. Scale bar, 20 µm.
Supplementary Video 4
Time-lapse (20 min intervals) live-cell video of F-actin dynamics and endosperm nuclei visualized by proFWA::Lifeact–Venus (cyan) and proFWA::H2B–mRuby2 (magenta), respectively, in Arabidopsis coenocytic endosperm of the control and 5 µM Lat B treatment (related to Fig. 2). The unspecific signal detected in the RFP channel in seed coat layers and dissected tissue debris is autofluorescence. Scale bar, 20 µm.
Supplementary Video 5
Time-lapse (20 min intervals) live-cell video (24 h in total) of F-actin dynamics and endosperm nuclei visualized by proFWA::Lifeact–Venus (cyan) and proFWA::H2B–mRuby2 (magenta), respectively, in DN-ACTIN (related to Fig. 3). The unspecific signal detected in the RFP channel in seed coat layers and dissected tissue debris is autofluorescence. Scale bar, 20 µm.
Supplementary Video 6
Time-lapse (20 min intervals) live-cell video (44 h in total) of F-actin dynamics and endosperm nuclei visualized by proFWA::Lifeact–Venus (cyan) and proFWA::H2B–mRuby2 (magenta), respectively, in OX-ACTIN (related to Fig. 3). The unspecific signal detected in the RFP channel in seed coat layers and dissected tissue debris is autofluorescence. Scale bar, 20 µm.
Supplementary Video 7
3D structure of coenocytic endosperm showing MTs visualized by proFWA::TagRFP–TUA5 (yellow) in Arabidopsis coenocytic endosperm (related to Fig. 4).
Supplementary Video 8
Time-lapse (20 min intervals) live-cell video (24 h in total) of MT dynamics visualized by proFWA::TagRFP–TUA5 (yellow) in Arabidopsis coenocytic endosperm of the control, 10 µM oryzalin treatment (related to Fig. 5) and 5 μM Lat B treatment (related to Extended Data Fig. 7). The unspecific signal detected in the RFP channel in seed coat layers and dissected tissue debris is autofluorescence. Scale bar, 20 µm.
Supplementary Video 9
Time-lapse (20 min intervals) live-cell video (21 h in total) of F-actin dynamics and endosperm nuclei visualized by proFWA::Lifeact–Venus (cyan) and proFWA::H2B–mRuby2 (magenta), respectively, in Arabidopsis coenocytic endosperm of the control and 10 µM oryzalin treatment (related to Fig. 5). The unspecific signal detected in the RFP channel in seed coat layers and dissected tissue debris is autofluorescence. Scale bar, 20 µm.
Supplementary Video 10
Time-lapse (30 min intervals) live-cell video of MTs visualized by proFWA::TagRFP–TUA5 (yellow), F-actin visualized by proFWA::Lifeact–Venus (cyan) and endosperm nuclei visualized by proFWA::H2B–mRuby2 (magenta) in Arabidopsis coenocytic endosperm of the MT control and the MTs after oryzalin washout, and the F-actin control and F-actin after oryzalin washout (related to Fig. 5). The unspecific signal detected in the RFP channel in seed coat layers and dissected tissue debris is autofluorescence. Scale bar, 20 µm.
Supplementary Video 11
Individual confocal Z-plane images of the cellularized endosperm among the control, DN-ACTIN and OX-ACTIN. Scale bar, 50 µm.
Source data
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 9
Statistical source data.
Source Data Extended Data Fig. 10
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ali, M.F., Shin, J.M., Fatema, U. et al. Cellular dynamics of coenocytic endosperm development in Arabidopsis thaliana. Nat. Plants 9, 330–342 (2023). https://doi.org/10.1038/s41477-022-01331-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41477-022-01331-7
This article is cited by
-
An actin starry night regulates seed size
Nature Plants (2023)