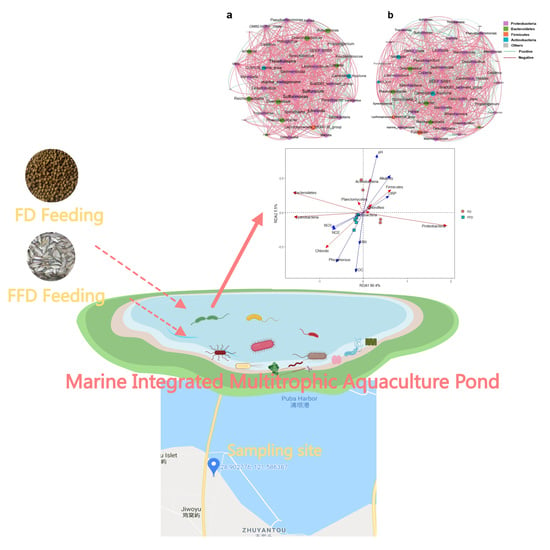
Feed Types Driven Differentiation of Microbial Community and Functionality in Marine Integrated Multitrophic Aquaculture System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Husbandry Environment
2.2. Water and Sediment Samples Collection
2.3. DNA Extraction and Sequencing
2.4. Bioinformatic Data Analysis
3. Results
3.1. Water Quality and Economic Performance
3.2. Microbial Community Diversity and Distribution
3.3. Microbial Composition in Water and Sediment
3.4. Correlations between Water Quality, Microbial Community and Functionality
4. Discussion
4.1. Water Quality and Its Effect on Microbial Distribution
4.2. Microbial Composition in Water and Sediment
4.3. Correlations between Water Quality and Microbial Community
4.4. Different Microbial Functionality Triggered by Feed Type
4.5. Investment and Benefits Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Naylor, R.L.; Goldburg, R.J.; Primavera, J.H.; Kautsky, N.; Beveridge, M.C.; Clay, J.; Folke, C.; Lubchenco, J.; Mooney, H.; Troell, M. Effect of aquaculture on world fish supplies. Nature 2000, 405, 1017–1024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- FAO. The State of World Fisheries and Aquaculture 2018—Meeting the Sustainable Development Goals; FAO: Rome, Italy, 2018. [Google Scholar]
- Chang, B.-V.; Liao, C.-S.; Chang, Y.-T.; Chao, W.-L.; Yeh, S.-L.; Kuo, D.-L.; Yang, C.-W. Investigation of a Farm-scale Multitrophic Recirculating Aquaculture System with the Addition of Rhodovulum sulfidophilum for Milkfish (Chanos chanos) Coastal Aquaculture. Sustainability 2019, 11, 1880. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Li, J.; Wang, Y.; Fu, L.; Fu, Y.; Li, B.; Jiao, B. Aquaculture industry in China: Current state, challenges, and outlook. Rev. Fish. Sci. 2011, 19, 187–200. [Google Scholar] [CrossRef]
- Chua Thia, E.; Paw, J.N.; Guarin, F.Y. The environmental impact of aquaculture and the effects of pollution on coastal aquaculture development in Southeast Asia. Mar. Pollut. Bull. 1989, 20, 335–343. [Google Scholar] [CrossRef]
- Bosma, R.H.; Verdegem, M.C.J. Sustainable aquaculture in ponds: Principles, practices and limits. Livest. Sci. 2011, 139, 58–68. [Google Scholar] [CrossRef]
- Troell, M.; Joyce, A.; Chopin, T.; Neori, A.; Buschmann, A.H.; Fang, J.-G. Ecological engineering in aquaculture—Potential for integrated multi-trophic aquaculture (IMTA) in marine offshore systems. Aquaculture 2009, 297, 1–9. [Google Scholar] [CrossRef]
- Zhang, J.; Kitazawa, D. Assessing the bio-mitigation effect of integrated multi-trophic aquaculture on marine environment by a numerical approach. Mar. Pollut. Bull. 2016, 110, 484–492. [Google Scholar] [CrossRef]
- Granada, L.; Sousa, N.; Lopes, S.; Lemos, M.F.L. Is integrated multitrophic aquaculture the solution to the sectors’ major challenges?—A review. Rev. Aquac. 2016, 8, 283–300. [Google Scholar] [CrossRef]
- Chávez-Crooker, P.; Obreque-Contreras, J. Bioremediation of aquaculture wastes. Curr. Opin. Biotechnol. 2010, 21, 313–317. [Google Scholar] [CrossRef]
- Moriarty, D.J.W. The role of microorganisms in aquaculture ponds. Aquaculture 1997, 151, 333–349. [Google Scholar] [CrossRef]
- Ruan, Y.J.; Deng, Y.L.; Guo, X.S.; Timmons, M.B.; Lu, H.F.; Han, Z.Y.; Ye, Z.Y.; Shi, M.M.; Zhu, S.M. Simultaneous ammonia and nitrate removal in an airlift reactor using poly(butylene succinate) as carbon source and biofilm carrier. Bioresour. Technol. 2016, 216. [Google Scholar] [CrossRef]
- Hargreaves, J.A. Photosynthetic suspended-growth systems in aquaculture. Aquacult. Eng. 2006, 34, 344–363. [Google Scholar] [CrossRef]
- Kong, D.; Li, W.; Deng, Y.; Ruan, Y.; Chen, G.; Yu, J.; Lin, F. Denitrification-Potential Evaluation and Nitrate-Removal-Pathway Analysis of Aerobic Denitrifier Strain Marinobacter hydrocarbonoclasticus RAD-2. Water 2018, 10, 1298. [Google Scholar] [CrossRef] [Green Version]
- Deng, Y.; Xu, X.; Yin, X.; Lu, H.; Chen, G.; Yu, J.; Ruan, Y. Effect of stock density on the microbial community in biofloc water and Pacific white shrimp (Litopenaeus vannamei) gut microbiota. Appl. Microbiol. Biotechnol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Wang, Z.; Chen, M.; Qu, Y.; Li, J.; Zhou, A.; Xie, S.; Zeng, F.; Zou, J. Microbiota comparison of Pacific white shrimp intestine and sediment at freshwater and marine cultured environment. Sci. Total Environ. 2019, 657, 1194–1204. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Pan, L.; Song, M.; Tian, C.; Gao, S. Microbiota assemblages of water, sediment, and intestine and their associations with environmental factors and shrimp physiological health. Appl. Microbiol. Biotechnol. 2018, 102, 8585–8598. [Google Scholar] [CrossRef]
- Zhou, Q.; Li, K.; Jun, X.; Bo, L. Role and functions of beneficial microorganisms in sustainable aquaculture. Bioresour. Technol. 2009, 100, 3780–3786. [Google Scholar] [CrossRef]
- Verdegem, M.C.J. Nutrient discharge from aquaculture operations in function of system design and production environment. Rev. Aquac. 2013, 5, 158–171. [Google Scholar] [CrossRef]
- Naylor, R.L.; Hardy, R.W.; Bureau, D.P.; Chiu, A.; Elliott, M.; Farrell, A.P.; Forster, I.; Gatlin, D.M.; Goldburg, R.J.; Hua, K.; et al. Feeding aquaculture in an era of finite resources. Proc. Natl. Acad. Sci. USA 2009, 106, 15103–15110. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Zhang, X.; Aweya, J.J.; Wang, S.; Hu, Z.; Li, S.; Wen, X. Formulated diet alters gut microbiota compositions in marine fish Nibea coibor and Nibea diacanthus. Aquac. Res. 2018, 50, 126–138. [Google Scholar] [CrossRef] [Green Version]
- Zhu, S.M.; Deng, Y.L.; Ruan, Y.J.; Guo, X.S.; Shi, M.M.; Shen, J.Z. Biological denitrification using poly(butylene succinate) as carbon source and biofilm carrier for recirculating aquaculture system effluent treatment. Bioresour. Technol. 2015, 192, 603–610. [Google Scholar] [CrossRef] [PubMed]
- SEPA. Water and Wastewater Monitoring Methods, 4th ed.; Chinese Environmental Science Publishing House: Beijing, China, 2002. [Google Scholar]
- Deng, Y.L.; Ruan, Y.J.; Zhu, S.M.; Guo, X.S.; Han, Z.Y.; Ye, Z.Y.; Liu, G.; Shi, M.M. The impact of DO and salinity on microbial community in poly(butylene succinate) denitrification reactors for recirculating aquaculture system wastewater treatment. AMB Express 2017, 7, 113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pena, A.G.; Goodrich, J.K.; Gordon, J.I. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, B.; Wang, H.; Dsouza, M.; Lou, J.; He, Y.; Dai, Z.; Brookes, P.C.; Xu, J.; Gilbert, J.A. Geographic patterns of co-occurrence network topological features for soil microbiota at continental scale in eastern China. ISME J. 2016, 10, 1891–1901. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Zhao, K.; Lv, X.; Su, W.; Dai, Z.; Gilbert, J.A.; Brookes, P.C.; Faust, K.; Xu, J. Genetic correlation network prediction of forest soil microbial functional organization. ISME J. 2018. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Li, K.; Han, H.; Zheng, Z.-X.; Bureau, D.P. Potential of using a blend of rendered animal protein ingredients to replace fish meal in practical diets for malabar grouper (Epinephelus malabricus). Aquaculture 2008, 281, 113–117. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, J.-L.; Li, K.; Bureau, D.P. Effects of dietary protein and energy levels on growth, feed utilization and body composition of cuneate drum (Nibea miichthioides). Aquaculture 2006, 252, 421–428. [Google Scholar] [CrossRef]
- Mello, B.L.; Alessi, A.M.; McQueen-Mason, S.; Bruce, N.C.; Polikarpov, I. Nutrient availability shapes the microbial community structure in sugarcane bagasse compost-derived consortia. Sci. Rep. 2016, 6, 38781. [Google Scholar] [CrossRef] [Green Version]
- Holmer, M.; Wildish, D.; Hargrave, B. Organic Enrichment from Marine Finfish Aquaculture and Effects on SedimentBiogeochemical Processes. In Environmental Effects of Marine Finfish Aquaculture; Hargrave, B.T., Ed.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 181–206. [Google Scholar]
- Cole, J.K.; Peacock, J.P.; Dodsworth, J.A.; Williams, A.J.; Thompson, D.B.; Dong, H.; Wu, G.; Hedlund, B.P. Sediment microbial communities in Great Boiling Spring are controlled by temperature and distinct from water communities. ISME J. 2013, 7, 718. [Google Scholar] [CrossRef] [Green Version]
- Deng, Y.; Ruan, Y.; Ma, B.; Timmons, M.B.; Lu, H.; Xu, X.; Zhao, H.; Yin, X. Multi-omics analysis reveals niche and fitness differences in typical denitrification microbial aggregations. Environ. Int. 2019, 132, 105085. [Google Scholar] [CrossRef] [PubMed]
- Hunter, E.M.; Mills, H.J.; Kostka, J.E. Microbial Community Diversity Associated with Carbon and Nitrogen Cycling in Permeable Shelf Sediments. Appl. Environ. Microb. 2006, 72, 5689–5701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oren, A. Saltern evaporation ponds as model systems for the study of primary production processes under hypersaline conditions. Aquat. Microb. Ecol. 2009, 56, 193–204. [Google Scholar] [CrossRef]
- Paerl, H.W.; Fulton, R.S.; Moisander, P.H.; Dyble, J. Harmful freshwater algal blooms, with an emphasis on cyanobacteria. Sci. World J. 2001, 1, 76–113. [Google Scholar] [CrossRef] [PubMed]
- Thomas, F.; Hehemann, J.H.; Rebuffet, E.; Czjzek, M.; Michel, G. Environmental and gut bacteroidetes: The food connection. Front. Microbiol. 2011, 2, 93. [Google Scholar] [CrossRef] [Green Version]
- Hwang, C.Y.; Bae, G.D.; Yih, W.; Cho, B.C. Marivita cryptomonadis gen. nov., sp. nov. and Marivita litorea sp. nov., of the family Rhodobacteraceae, isolated from marine habitats. Int. J. Syst. Evol. Microbiol. 2009, 59, 1568–1575. [Google Scholar] [CrossRef] [Green Version]
- Kang, I.; Lee, K.; Yang, S.-J.; Choi, A.; Kang, D.; Lee, Y.K.; Cho, J.-C. Genome sequence of “Candidatus Aquiluna” sp. strain IMCC13023, a marine member of the Actinobacteria isolated from an arctic fjord. J. Bacteriol. 2012, 194, 3550–3551. [Google Scholar] [CrossRef] [Green Version]
- Shen, L.D.; Wu, H.S.; Gao, Z.Q.; Ruan, Y.J.; Xu, X.H.; Li, J.; Ma, S.J.; Zheng, P.H. Evidence for anaerobic ammonium oxidation process in freshwater sediments of aquaculture ponds. Environ. Sci. Pollut. Res. 2016, 23, 1344–1352. [Google Scholar] [CrossRef]
- Miura, Y.; Watanabe, Y.; Okabe, S. Significance of Chloroflexi in Performance of Submerged Membrane Bioreactors (MBR) Treating Municipal Wastewater. Environ. Sci. Technol. 2007, 41, 7787–7794. [Google Scholar] [CrossRef]
- Kragelund, C.; Caterina, L.; Borger, A.; Thelen, K.; Eikelboom, D.; Tandoi, V.; Kong, Y.; Van Der Waarde, J.; Krooneman, J.; Rossetti, S.; et al. Identity, abundance and ecophysiology of filamentous Chloroflexi species present in activated sludge treatment plants. FEMS Microbiol. Ecol. 2007, 59, 671–682. [Google Scholar] [CrossRef] [Green Version]
- Hug, L.A.; Castelle, C.J.; Wrighton, K.C.; Thomas, B.C.; Sharon, I.; Frischkorn, K.R.; Williams, K.H.; Tringe, S.G.; Banfield, J.F. Community genomic analyses constrain the distribution of metabolic traits across the Chloroflexi phylum and indicate roles in sediment carbon cycling. Microbiome 2013, 1, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inagaki, F.; Takai, K.; Nealson, K.H.; Horikoshi, K. Sulfurovum lithotrophicum gen. nov., sp. nov., a novel sulfur-oxidizing chemolithoautotroph within the ε-Proteobacteria isolated from Okinawa Trough hydrothermal sediments. Int. J. Syst. Evol. Microbiol. 2004, 54, 1477–1482. [Google Scholar] [CrossRef] [PubMed]
- Cole, J.J. Interactions between bacteria and algae in aquatic ecosystems. Annu. Rev. Ecol. Syst. 1982, 13, 291–314. [Google Scholar] [CrossRef]
- Poli, M.A.; Legarda, E.C.; de Lorenzo, M.A.; Pinheiro, I.; Martins, M.A.; Seiffert, W.Q.; do Nascimento Vieira, F. Integrated multitrophic aquaculture applied to shrimp rearing in a biofloc system. Aquaculture 2019, 511, 734274. [Google Scholar] [CrossRef]
- Zhu, S.M.; Shi, M.M.; Ruan, Y.J.; Guo, X.S.; Ye, Z.Y.; Han, Z.Y.; Deng, Y.L.; Liu, G. Applications of computational fluid dynamics to modeling hydrodynamics in tilapia rearing tank of Recirculating Biofloc Technology system. Aquacult. Eng. 2016, 74, 120–130. [Google Scholar] [CrossRef]
- Thompson, K.J.; Simister, R.L.; Hahn, A.S.; Hallam, S.J.; Crowe, S.A. Nutrient Acquisition and the Metabolic Potential of Photoferrotrophic Chlorobi. Front. Microbiol. 2017, 8. [Google Scholar] [CrossRef] [Green Version]
- Zehr, J.P. Nitrogen fixation by marine cyanobacteria. Trends Microbiol. 2011, 19, 162–173. [Google Scholar] [CrossRef]
- Dauda, A.B.; Ajadi, A.; Tola-Fabunmi, A.S.; Akinwole, A.O. Waste production in aquaculture: Sources, components and managements in different culture systems. Aquac. Fish. 2019, 4, 81–88. [Google Scholar] [CrossRef]
- Lauber, C.L.; Hamady, M.; Knight, R.; Fierer, N. Pyrosequencing-Based Assessment of Soil pH as a Predictor of Soil Bacterial Community Structure at the Continental Scale. Appl. Environ. Microb. 2009, 75, 5111–5120. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.-J.; Chen, C.; Guan, X.; Yuan, Y.; Wang, A.-J.; Lee, D.-J.; Zhang, Z.-F.; Zhang, J.; Zhong, Y.-J.; Ren, N.-Q. Performance and microbial community analysis of a microaerophilic sulfate and nitrate co-reduction system. Chem. Eng. J. 2017, 330, 63–70. [Google Scholar] [CrossRef]
- Toranzo, A.E.; Magariños, B.; Romalde, J.L. A review of the main bacterial fish diseases in mariculture systems. Aquaculture 2005, 246, 37–61. [Google Scholar] [CrossRef]
- Bowman, J. Bioactive compound synthetic capacity and ecological significance of marine bacterial genus Pseudoalteromonas. Mar. Drugs 2007, 5, 220–241. [Google Scholar] [CrossRef] [PubMed]
- Dawood, M.A.O.; Koshio, S.; Abdel-Daim, M.M.; Van Doan, H. Probiotic application for sustainable aquaculture. Rev. Aquac. 2018. [Google Scholar] [CrossRef]
- Zhu, Y.-G.; Zhao, Y.; Zhu, D.; Gillings, M.; Penuelas, J.; Ok, Y.S.; Capon, A.; Banwart, S. Soil biota, antimicrobial resistance and planetary health. Environ. Int. 2019, 131, 105059. [Google Scholar] [CrossRef] [PubMed]
- Retnam, A.; Zakaria, M.P.; Juahir, H.; Aris, A.Z.; Zali, M.A.; Kasim, M.F. Chemometric techniques in distribution, characterisation and source apportionment of polycyclic aromatic hydrocarbons (PAHS) in aquaculture sediments in Malaysia. Mar. Pollut. Bull. 2013, 69, 55–66. [Google Scholar] [CrossRef] [PubMed]






| Water Quality Parameters | FFD | FD | Significance |
|---|---|---|---|
| TAN (mg L−1) | 0.34 ± 0.06 | 0.29 ± 0.09 | ns |
| NO2−-N (mg L−1) | 0.06 ± 0.01 | 0.03 ± 0.01 | ** |
| NO3−-N (mg L−1) | 0.39 ± 0.07 | 0.24 ± 0.05 | ** |
| DRP (mg L−1) | 0.09 ± 0.01 | 0.03 ± 0.01 | ** |
| TOC (mg L−1) | 14.8 ± 2.02 | 10.19 ± 2.76 | ** |
| pH | 8.02 ± 0.07 | 8.26 ± 0.17 | * |
| ORP (mV) | 264.2 ± 40.67 | 305.8 ± 28.21 | ns |
| Alkalinity (mg L−1) | 129.8 ± 16.45 | 164.2 ± 10.31 | * |
| Phylum | FFD | FD | p Value | |||
|---|---|---|---|---|---|---|
| Average (%) | Sd | Average (%) | Sd | |||
| Sediment | Chloroflexi | 1.13 × 10−2 | 2.85 × 10−3 | 1.88 × 10−2 | 6.06 × 10−3 | 0.04698 |
| Spirochaetae | 1.04 × 10−2 | 7.33 × 10−4 | 6.79 × 10−3 | 2.97 × 10−3 | 0.04991 | |
| TM6 | 2.98 × 10−3 | 1.04 × 10−3 | 1.47 × 10−3 | 6.03 × 10−4 | 0.02828 | |
| Lentisphaerae | 5.86 × 10−4 | 3.08 × 10−4 | 1.29 × 10−4 | 8.44 × 10−5 | 0.02700 | |
| WCHB1-60 | 1.17 × 10−4 | 8.55 × 10−5 | 5.86 × 10−6 | 1.31 × 10−5 | 0.04258 | |
| Water | Bacteroidetes | 2.24 × 10−1 | 1.91 × 10−2 | 1.36 × 10−1 | 4.92 × 10−2 | 0.01314 |
| Cyanobacteria | 2.73 × 10−1 | 2.99 × 10−2 | 1.65 × 10−1 | 4.36 × 10−2 | 0.00256 | |
| Firmicutes | 3.09 × 10−3 | 1.71 × 10−3 | 1.21 × 10−2 | 2.78 × 10−3 | 0.00054 | |
| Chlorobi | 2.10 × 10−2 | 4.23 × 10−3 | 3.76 × 10−3 | 1.68 × 10−3 | 0.00030 | |
| Tenericutes | 8.73 × 10−4 | 1.08 × 10−4 | 4.34 × 10−4 | 3.19 × 10−4 | 0.03393 | |
| Cloacimonetes | 8.97 × 10−4 | 3.57 × 10−4 | 3.46 × 10−4 | 3.08 × 10−4 | 0.03147 | |
| Lentisphaerae | 3.99 × 10−4 | 2.88 × 10−4 | 1.17 × 10−5 | 1.61 × 10−5 | 0.03981 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, Y.; Zhou, F.; Ruan, Y.; Ma, B.; Ding, X.; Yue, X.; Ma, W.; Yin, X. Feed Types Driven Differentiation of Microbial Community and Functionality in Marine Integrated Multitrophic Aquaculture System. Water 2020, 12, 95. https://doi.org/10.3390/w12010095
Deng Y, Zhou F, Ruan Y, Ma B, Ding X, Yue X, Ma W, Yin X. Feed Types Driven Differentiation of Microbial Community and Functionality in Marine Integrated Multitrophic Aquaculture System. Water. 2020; 12(1):95. https://doi.org/10.3390/w12010095
Chicago/Turabian StyleDeng, Yale, Fan Zhou, Yunjie Ruan, Bin Ma, Xueyan Ding, Xiaomei Yue, Wenjun Ma, and Xuwang Yin. 2020. "Feed Types Driven Differentiation of Microbial Community and Functionality in Marine Integrated Multitrophic Aquaculture System" Water 12, no. 1: 95. https://doi.org/10.3390/w12010095