Nanoscale Zero Valent Iron Supported by Biomass-Activated Carbon for Highly Efficient Total Chromium Removal from Electroplating Wastewater
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Preparation of BC-nZVI Material
2.3. Characterization
2.4. Removal Mechanism and Performance of Total Cr by BC-nZVI
2.4.1. Batch Experiments
2.4.2. Kinetic Analysis of Total Cr Removal
3. Results and Discussions
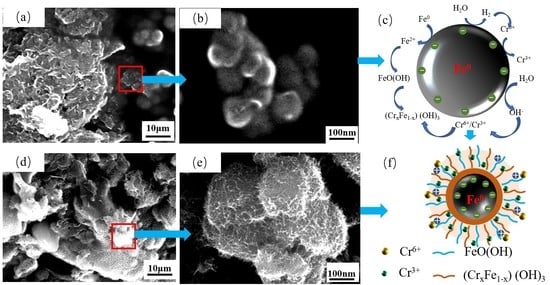
3.1. Synthesis and Characterization of Materials
3.2. Degradation Mechanism for Reduction–Adsorption of Cr on BC-nZVI
3.3. Kinetic of Total Cr Removal by BC-nZVI
3.4. Effect of the Operated Factors on Total Cr Removal
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mandiwana, K.L.; Resane, T.; Panichev, N.; Ngobeni, P. The application of tree bark as bio-indicator for the assessment of Cr(VI) in air pollution. J. Hazard. Mater. 2006, 137, 1241–1245. [Google Scholar] [CrossRef] [PubMed]
- Javed, M.; Usmani, N. Assessment of heavy metal (Cu, Ni, Fe, Co, Mn, Cr, Zn) pollution in effluent dominated rivulet water and their effect on glycogen metabolism and histology of mastacembelus armatus. SpringerPlus 2013, 2, 390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dingtian, Y.; Guoxin, S.; Dongjie, S.; Weimin, C. The resistant reaction of brasenia schreberi winter-bud to Cr6+ pollution. J. Lake Sci. 2001, 13, 169–174. [Google Scholar] [CrossRef] [Green Version]
- Lin, W.J. Characteristics and control of Cr pollution in electroplating wastewater. Environ. Sci. Technol. 2010, 33, 156–158. [Google Scholar] [CrossRef]
- Di Palma, L.; Verdone, N.; Vilardi, G. Kinetic Modeling of Cr(VI) Reduction by nZVI in Soil: The Influence of Organic Matter and Manganese Oxide. Bull. Environ. Contam. Toxicol. 2018, 101, 692–697. [Google Scholar] [CrossRef]
- Cui, J.; Wang, E.; Hou, Z.; Zhou, R.; Jiao, X. Removal of Chromium(VI) from Groundwater Using Oil Shale Ash Supported Nanoscaled Zero-valent Iron. Chem. Res. Chin. Univ. 2018, 34, 546–551. [Google Scholar] [CrossRef]
- Zhu, Y.; Jin, Y.; Chang, K.; Chen, Z.; Li, X.; Wu, X.; Jin, C.; Ye, F.; Shen, R.; Dong, W.; et al. Use of molybdenum disulfide nanosheets embellished nanoiron for effective capture of chromium (VI) ions from aqueous solution. J. Mol. Liq. 2018, 259, 376–383. [Google Scholar] [CrossRef]
- Zhu, F.; Li, L.; Ren, W.; Deng, X.; Liu, T. Effect of pH, temperature, humic acid and coexisting anions on reduction of Cr(VI) in the soil leachate by nZVI/Ni bimetal material. Environ. Pollut. 2017, 227, 444–450. [Google Scholar] [CrossRef]
- Wu, B.; Peng, D.; Hou, S.; Tang, B.; Wang, C.; Xu, H. Dynamic study of Cr(VI) removal performance and mechanism from water using multilayer material coated nanoscale zerovalent iron. Environ. Pollut. 2018, 240, 717–724. [Google Scholar] [CrossRef]
- Zhu, S.; Huang, X.; Wang, D.; Wang, L.; Ma, F. Enhanced hexavalent chromium removal performance and stabilization by magnetic iron nanoparticles assisted biochar in aqueous solution: Mechanisms and application potential. Chemosphere 2018, 207, 50–59. [Google Scholar] [CrossRef]
- Xing, Y.; Chen, X.; Wang, D. Electrically regenerated ion exchange for removal and recovery of Cr(VI) from wastewater. Environ. Sci. Technol. 2007, 41, 1439–1443. [Google Scholar] [CrossRef] [PubMed]
- Ren, T.; He, P.; Niu, W.; Wu, Y.; Ai, L.; Gou, X. Synthesis of α-Fe2O3 nanofibers for applications in removal and recovery of Cr(VI) from wastewater. Environ. Sci. Pollut. Res. Int. 2013, 20, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Priyantha, N.; Bandaranayaka, A. Interaction of Cr(VI) species with thermally treated brick clay. Environ. Sci. Pollut. Res. 2011, 18, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Liu, L.; Zhou, L.; Zhang, S. Electrokinetic removal of chromium from chromite ore-processing residue using graphite particle-supported nanoscale zero-valent iron as the three-dimensional electrode. Chem. Eng. J. 2018, 350, 1022–1034. [Google Scholar] [CrossRef]
- Sulaymon, A.H.; Ebrahim, S.E.; Mohammed-Ridha, M.J. Equilibrium, kinetic, and thermodynamic biosorption of Pb(II), Cr(III), and Cd(II) ions by dead anaerobic biomass from synthetic wastewater. Environ. Sci. Pollut. Res. Int. 2013, 20, 175–187. [Google Scholar] [CrossRef]
- Feng, H.; Tang, L.; Tang, J.; Zeng, G.; Dong, H.; Deng, Y.; Zhou, Y. Cu-Doped Fe@Fe2O3 core–shell nanoparticle shifted oxygen reduction pathway for high-efficiency arsenic removal in smelting wastewater. Environ. Sci. Nano 2018, 5, 1595–1607. [Google Scholar] [CrossRef]
- Ghosh, S.; Remita, H.; Basu, R.N. Visible-light-induced reduction of Cr(VI) by PDPB-ZnO nanohybrids and its photo-electrochemical response. Appl. Catal. B Environ. 2018, 239, 362–372. [Google Scholar] [CrossRef]
- Guo, X.; Yang, Z.; Dong, H.; Guan, X.; Ren, Q.; Lv, X.; Jin, X. Simple combination of oxidants with zero-valent-iron (ZVI) achieved very rapid and highly efficient removal of heavy metals from water. Water Res. 2016, 88, 671–680. [Google Scholar] [CrossRef]
- Jia, Z.; Shu, Y.; Huang, R.; Liu, J.; Liu, L. Enhanced reactivity of nZVI embedded into super macroporous cryogels for highly efficient Cr(VI) and total Cr removal from aqueous solution. Chemosphere 2018, 199, 232–242. [Google Scholar] [CrossRef]
- Liu, X.; Lai, D.; Wang, Y. Performance of Pb(II) removal by an activated carbon supported nanoscale zero-valent iron composite at ultralow iron content. J. Hazard. Mater. 2019, 361, 37–48. [Google Scholar] [CrossRef]
- Soroosh, M.; Hyeunhwan, A.; Dongwon, C.; Jaeyun, M. Activated carbon impregnated by zero-valent iron nanoparticles (AC/nZVI) optimized for simultaneous adsorption and reduction of aqueous hexavalent chromium: Material characterizations and kinetic studies. Chem. Eng. J. 2018. [Google Scholar] [CrossRef]
- Sheng, G.; Shao, X.; Li, Y.; Li, J.; Dong, H.; Cheng, W.; Huang, Y. Enhanced removal of uranium (VI) by nanoscale zerovalent iron supported on Na-bentonite and an investigation of mechanism. J. Phys. Chem. A 2014, 118, 2952–2958. [Google Scholar] [CrossRef] [PubMed]
- Vilardi, G.; Mpouras, T.; Dermatas, D.; Verdone, N.; Polydera, A.; Di Palma, L. Nanomaterials application for heavy metals recovery from polluted water: The combination of nano zero-valent iron and carbon nanotubes. Competitive adsorption non-linear modeling. Chemosphere 2018, 201, 716–729. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wang, D.P. Preparation of Biomass Activated Carbon Supported Nanoscale Zero-Valent Iron (nZVI) and Its Application in Decolorization of Methyl Orange from Aqueous Solution. Water 2019, 11, 1671. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Wei, D.; Li, Q.; Wang, X.; Yu, S.; Liu, L. Macroscopic and microscopic investigation of Cr(VI) immobilization by nanoscaled zero-valent iron supported zeolite MCM-41 via batch, visual, xps and exafs techniques. J. Clean. Prod. 2018, 181, 745–752. [Google Scholar] [CrossRef]
- Lv, X.; Xu, J.; Jiang, G.; Tang, J.; Xu, X. Highly active nanoscale zero-valent iron (nZVI)–Fe3O4 nanocomposites for the removal of chromium(VI) from aqueous solutions. J. Colloid Interface Sci. 2012, 369, 460–469. [Google Scholar] [CrossRef]
- Xia, X.; Ling, L.; Zhang, W.X. Genesis of pure Se (0) nano- and micro-structures in wastewater with nanoscale zero-valent iron (nZVI). Environ. Sci. Nano 2017, 4, 52–59. [Google Scholar] [CrossRef]
- Watts, M.P.; Coker, V.S.; Parry, S.A.; Pattrick, R.A.D.; Thomas, R.A.P.; Kalin, R.; Lloyd, J.R. Biogenic nano-magnetite and nano-zero valent iron treatment of alkaline Cr(VI) leachate and chromite ore processing residue. Appl. Geochem. 2015, 54, 27–42. [Google Scholar] [CrossRef]
- Li, S.; Wang, W.; Yan, W.; Zhang, W.X. Nanoscale zero-valent iron (nZVI) for the treatment of concentrated Cu (II) wastewater: A field demonstration. Environ. Sci. Process. Impacts 2014, 16, 524–533. [Google Scholar] [CrossRef]
- Li, X.; Ai, L.; Jiang, J. Nanoscale zerovalent iron decorated on graphene nanosheets for Cr(VI) removal from aqueous solution: Surface corrosion retard induced the enhanced performance. Chem. Eng. J. 2016, 288, 789–797. [Google Scholar] [CrossRef]
- Alidokht, L.; Khataee, A.R.; Reyhanitabar, A.; Oustan, S. Reductive removal of Cr(VI) by starch-stabilized fe0 nanoparticles in aqueous solution. Desalination 2011, 270, 105–110. [Google Scholar] [CrossRef]
- Montesinos, V.N.; Quici, N.; Litter, M.I. Visible light enhanced Cr(VI) removal from aqueous solution by nanoparticulated zerovalent iron. Catal. Commun. 2014, 46, 57–60. [Google Scholar] [CrossRef]










| C0 (mg/L) | qe (mg/g) | PFO | PSO | ||||
|---|---|---|---|---|---|---|---|
| qe (mg/g) | k1 (min−1) | R2 | qe (mg/g) | k2 (g/(mg·min)) | R2 | ||
| nZVI | 76.64 | 78.47 | 0.0096 | 0.7955 | 77.78 | 0.0016 | 0.9477 |
| BC-nZVI (One Day) | 138.76 | 143.53 | 0.0201 | 0.8326 | 136.47 | 0.0009 | 0.9995 |
| BC-nZVI (One Week) | 105.96 | 96.76 | 0.0071 | 0.8271 | 101.16 | 0.0011 | 0.9704 |
| BC-nZVI (One Month) | 48.88 | 42.47 | 0.0012 | 0.8174 | 49.94 | 0.0026 | 0.9979 |
| Material | Removal Capacity | Source |
|---|---|---|
| PSA- nZVI (poly sodium acrylate) | 138.80 mg/g | Jia et al., 2018 [19] |
| GN-nZVI (graphene nanosheets) | 21.72 mg/g | Li et al. [30] |
| S-nZVI (Starch-stabilized) | 20.16 mg/g | Alidokht et al. [31] |
| nZVI | 47.2 mg/g | Montesinos et al. [32] |
| nZVI | 77.78 mg/g | This study |
| BC-nZVI | 136.47 mg/g | This study |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, B.; Zhu, B.-H.; Wang, X.; You, S.-B. Nanoscale Zero Valent Iron Supported by Biomass-Activated Carbon for Highly Efficient Total Chromium Removal from Electroplating Wastewater. Water 2020, 12, 89. https://doi.org/10.3390/w12010089
Zhang B, Zhu B-H, Wang X, You S-B. Nanoscale Zero Valent Iron Supported by Biomass-Activated Carbon for Highly Efficient Total Chromium Removal from Electroplating Wastewater. Water. 2020; 12(1):89. https://doi.org/10.3390/w12010089
Chicago/Turabian StyleZhang, Bo, Bo-Hong Zhu, Xiong Wang, and Song-Bai You. 2020. "Nanoscale Zero Valent Iron Supported by Biomass-Activated Carbon for Highly Efficient Total Chromium Removal from Electroplating Wastewater" Water 12, no. 1: 89. https://doi.org/10.3390/w12010089