1. Introduction
Increasing antimony (Sb) contaminations pose a great threat to the environment and human beings, and have attracted increasing attention worldwide [
1]. Besides natural activities, human activities, especially mining, dying, and textile industries, have aggravated the pollution and spread of Sb. Sb is highly toxic to human health, causing damage to the skin, eyes, lungs, stomach, liver, kidneys, heart, and nervous system [
2]. Sb has been declared a high-priority pollutant of interest by the United States Environmental Protection Agency (USEPA) and European Union (EU). The maximum allowable concentration of Sb in water is regulated as 6, 10, and 5 μg·L
−1 by the USEPA, EU, and the World Health Organization (WHO), respectively [
3,
4,
5].
Given the high toxicity and severe pollution of Sb, many technologies have been developed to remove Sb from water in recent years, such as coagulation [
6,
7], adsorption [
8,
9], membrane [
10], and electro-coagulation [
11] related technologies. For example, many adsorbents were applied to remove Sb, such as goethite, diatomite, and Fe-Mn binary oxide [
12]. Peatlands and constructed wetlands were investigated in regard to the removal of Sb from mining waste water via adsorption processes [
13,
14]. Among the developed technologies, the coagulation method remains popular worldwide due to its low cost, high efficiency, simple design and operation, and good matching with other water treatment equipment [
15,
16]. In addition, coagulation can achieve in situ removal of Sb in the case of emergency pollution incidents. Sb removal by coagulation with iron salts and aluminum salts was investigated, and the results revealed that ferric chloride (FC) exhibited better performance than poly-aluminum chloride for Sb removal, indicating that iron-based coagulants exhibited good performance in Sb removal [
17]. Additionally, iron-based coagulants were demonstrated to exhibit good performance in Sb removal. The adsorption of formed hydrous ferric oxide (HFO) was found to play an important role in Sb removal [
18,
19]. However, the performance of different iron-based coagulants for Sb removal, such as polymeric ferric sulfate (PFS), FC, and ferrous sulfate (FeSO
4), still remains to be explored.
Sb is a metalloid, the fourth element of Group VA in the periodic table. Given its natural and anthropogenic activities, Sb has been found in soil and water environments. Sb(III) and Sb(V) are the predominant states of Sb that exist in aerobic environments [
20,
21,
22]. Under mildly acidic, neutral, and alkaline conditions, Sb(OH)
3 and Sb(OH)
6− are the dominant species for Sb(III) and Sb(V), respectively. Sb(III) is more toxic than Sb(V), but the solubility of the latter is higher than that of the former [
2]. Guo et al. found that Sb(III) was easier to remove by coagulation with FC than Sb(V) [
18]. In natural aqueous environments, many co-existing matters, including competing ions and organic matters, could interfere with the removal of Sb by coagulation. Moreover, the oxidization process can affect the state and removal of Sb in water by coagulation [
23]. For example, the addition of KMnO
4 and aeration process, the common water treatment process, can oxidize Sb(III) to Sb(V) [
24,
25].
In the present work, the effects of solution pH, coagulant types, and dose on the removal of Sb by Fe(III)- and Fe(II)-based coagulants were investigated. In addition, the effects of competing matters, as well as oxidation, for Sb removal by coagulation were explored. The adsorption isotherms and mechanisms were also studied.
3. Results and Discussion
3.1. Effects of pH
As shown in
Figure 1a, 84%, 88%, and 15% of Sb(III) was removed by PFS, FC, and FeSO
4, respectively at a pH of 3. When the pH was increased to 5, the highest removal rates of Sb(III) by using PFS, FC, and FeSO
4 were obtained (95%, 94%, and 60%, respectively). The Sb(III) removal rate by using PFS and FC remained higher than 85% within a wide pH range of 3 to 9. However, for FeSO
4, the removal rate decreased remarkably when the pH was increased from 5 to 10. As shown in
Figure 1b, the highest removal rate of Sb(V) was 92% and 93% at pH 5 with PFS and FC, respectively, and 79% at pH 6 with FeSO
4. When the pH was increased from 3 to 5, the removal rate of Sb(V) increased slightly by using PFS and FC; however, a steep increase of the Sb(V) removal rate was obtained (4% to 75%) by using FeSO
4. When the pH values increased from 5 to 6, the removal rate of Sb(V) by using the three coagulants changed slightly. When the pH was further increased from 6 to 10, the removal rate of Sb(V) decreased to 12%, 15%, and 7% for PFS, FC, and FeSO
4, respectively. The optimum pH value was set at pH 5 for other batches.
Within a broad pH range of 3 to 9, effective Sb(III) removal was obtained with PFS and FC. However, the optimum pH value for Sb(V) removal ranged from 5 to 6. For PFS and FC, the removal efficiency of Sb(III) was higher than that of Sb(V). However, for FeSO
4, the removal efficiency of Sb(III) was lower than that of Sb(V). As the pH value increased, the zeta potential of PFS, FC, and FeSO
4 decreased (
Figure 1c). When the pH value was below 7, the formed flocs was positively charged. When the iron-based coagulants were added into water at the optimum pH of 5, Fe(III) ions were hydrolyzed to form HFO. During agitation, the positively charged HFO adsorbed the neutrally charged Sb(III) or negatively charged Sb (V), resulting in the electrical neutralization and compression of the electric double layer for colloids. As a result, the HFO colloid became unstable and larger HFO flocculates were formed, precipitated, and separated from water, achieving the removal of antimony. The electrostatic interaction between the positively charged flocs and negatively charged Sb(OH)
6− promoted the removal of Sb(V). However, when the pH value was below 3, the hydrolysis of iron-based coagulants decreased, which inhibited the removal of Sb.
In the coagulation process for Sb removal with iron-based coagulants, the active HFO flocs with many adsorption sites were formed due to the hydrolysis of iron ions in solution [
10]. The pH value of the solution affected the properties of HFO flocs and the state of Sb in solution, which further affected the removal of Sb by coagulation. At pH 5, Fe(OH)
2+ and Fe(OH)
2+ were the predominant Fe(III) species other than Fe(OH)
3. With the further increase of pH, the cationic Fe species decreased, and anionic Fe species such as Fe(OH)
4− increased [
17]. In solution, Sb(V) existed as soluble anion Sb(OH)
6−. The electrostatic interaction between Sb(OH)
6− and Fe(III) species facilitated the combination of HFO–Sb(V), which was affected by the pH value. Hence, the removal of Sb(V) by coagulation was affected. However, Sb(III) existed as a neutral molecule in water. The interaction between neutral Sb(III) and HFO flocs was due to chemical bonding, which was affected slightly by pH values of the solution. The low solubility of Sb(III) species facilitated Sb(III) removal [
10]. Contrary to the effective Sb removal by using PFS and FC, the removal efficiency by using FeSO
4 was relatively low. Although the removal efficiency of Sb(V) with FeSO
4 was higher than that of Sb(III), it was still lower than that of Sb(V) with Fe(III)-based coagulants.
3.2. Effects of Coagulant Dose
Figure 2 shows that as the coagulant concentration increased, the removal rates of Sb(III) and Sb(V) increased until equilibrium was reached. Both PFS and FC exhibited better removal performance for Sb than FeSO
4. Approximately 20 mg·L
−1 of PFS and FC were required to reduce the concentration of Sb(III) below 5 μg·L
−1, in compliance with the WHO drinking water quality standard. Approximately 30 mg·L
−1 of PFS and FC were required to decrease the concentration of Sb(V) below 5 μg·L
−1, indicating that Sb(V) was more difficult to remove than Sb(III) by using PFS and FC. By coagulating with FeSO
4, the remaining concentrations of Sb(III) and Sb(V) were higher than 5 μg·L
−1, which did not meet the standard.
Furthermore, Sb removal experiments were carried out using natural water obtained from Taihu Lake, Jiangsu Province, China (
Figure S1). The Chemical Oxygen Demand (COD) in water was 42 mg·L
−1. PFS was applied to remove Sb, and the initial concentrations of Sb(III) and Sb(V) were 104 and 116 μg·L
−1. Both Sb(III) and Sb(V) were effectively removed at the PFS dosage of 20 mg·L
−1, and the concentration of residual Sb was below 5 μg·L
−1, meeting the WHO drinking water standard.
After coagulation, there were possibly some iron ions left in the water. Most iron ions could be precipitated as Fe(OH)3 in neutral water or removed in the subsequent treatment steps. The low toxicity and biocompatibility of iron-based coagulants ensured the safety of the treated water.
For the practical emergency treatment of Sb pollution in a lake, there are two main ways of using coagulation, namely, in-situ emergency treatment and off-site emergency treatment. The in-situ treatment involves adding chemicals into the lake directly. The pollutants will be deposited along with the sediment at the bottom of the lake, but there is a risk that the sediments will possibly be stirred up again. The off-site treatment involves pumping lake water into a sewage treatment station or facility for treatment. The treated water can be discharged back into the lake. The resulting sludge can be separated through a sedimentation tank, and then tested, treated, or comprehensively utilized.
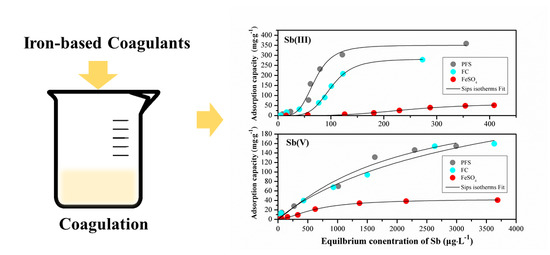
3.3. Adsorption Isotherms
Adsorption by the formed HFO flocs during the coagulation process was the main routine for Sb removal [
18]. The adsorption isotherms were applied to describe the adsorption behavior of Sb by HFO flocs in the coagulation, including Langmuir, Freundlich, and Sips isotherms. The Langmuir isotherm illustrates the adsorption behavior of an ideal adsorption system with homogeneous surface of the adsorbent. However, the results of simulated data via Langmuir isotherm were unreasonable. The Freundlich isotherm explains heterogeneous adsorption behavior, which indicates that different adsorption energies exist in different surface sites [
26]. The Sips isotherm is derived from the Langmuir and Freundlich isotherms. The result of adsorption capacity for Sb fitted well with the Sips adsorption isotherm. All the parameters of equations, as shown in
Table 1, were derived from the nonlinear least-squares regression of the experimental data.
Figure 3a shows that with the increase of residual Sb concentration, the adsorption capacity increased until reaching an equilibrium. The fitted Sips isotherms revealed that the behavior of Sb adsorption was related to heterogeneous adsorption at low concentration but was similar to the homogenous adsorption at high concentration [
26]. The maximum simulated adsorption capacity was 350 mg·g
−1 with PFS, 356 mg·g
−1 with FC, and 61 mg·g
−1 with FeSO
4 for Sb(III), and 264 mg·g
−1 with PFS, 306 mg·g
−1 with FC, and 43 mg·g
−1 with FeSO
4 for Sb(V), which were higher than many other iron-based adsorbents [
27,
28]. It indicates that the coagulation is potential at removing Sb at high initial concentrations. As shown in
Figure 3b, PFS and FC exhibited similar maximum adsorption behavior. The removal performance of Sb(V) with both PFS and FC was better than that with FeSO
4. The adsorption capacities for Sb(V) with iron-based coagulants were much lower than those of Sb(III), and the equilibrium concentration of Sb was higher than that of Sb(III).
3.4. Effects of Co-Existing Substances
In a natural aqueous system, many constitutes may affect the removal of Sb, such as HA and anions [
16]. The HA is connected with many functional groups, such as carboxyl and hydroxyl groups, which commonly exist in the natural environment.
Figure 4 shows the effects of HA, Cl
−, F
−, NO
3−, HCO
3−, SO
42−, and PO
43− on the removal of Sb by coagulation with FPS. In the presence of Cl
−, F
−, NO
3−, and SO
42−, the effects on the removal rate of Sb by coagulation with 10 mg·L
−1 of PFS were negligible. With the presence of HCO
3−, the removal rate of Sb(V) by coagulation was slightly decreased because the hydrolysis of HCO
3− decreased the pH of the solution and affected the removal of Sb(V). However, with the presence of PO
43−, the removal of Sb(III) and Sb(V) was decreased remarkably, which was ascribed to the competition of adsorption sites between Sb and phosphate ions due to the similar tetrahedral structure and s
2p
3 outer electronic orbit [
29]. HA also inhibited the removal of Sb(III) and Sb(V) remarkably. With the presence of HA, the removal rates of Sb(III) by using PFS, FC, and FeSO
4 decreased by 21.01%, 27.13%, and 19.79%, respectively, and those of Sb(V) decreased by 31.67%, 36.10%, and 31.75% respectively. On the one hand, HA was competitive with Sb on the adsorption sites of coagulants. On the other hand, reactive functional groups of HA, such as phenolic and carboxylic acid, facilitated the combination of Sb and HA, which increased the solubility of Sb and hence inhibited the removal of Sb [
16,
18].
3.5. Effects of Oxidation on Sb Removal
In a naturally aerobic environment, many oxidizing substances may oxidize Sb(III) to Sb(V), which affects the mobility and removal of Sb [
25,
30]. In this study, the effects of KMnO
4 and aeration on the removal of Sb by coagulation with PFS of 10 mg·L
−1 were investigated. Before coagulation, different concentrations of KMnO
4 were applied for pre-oxidization, and the aeration process was conducted for different times.
Figure 5a shows that the removal efficiency of Sb(III) decreased remarkably from 95% to 35% with the presence of 1 mg·L
−1 KMnO
4, which was ascribed to the oxidization of Sb(III) by KMnO
4 and interference of MnO
4− on the competition for the adsorption sites. When the KMnO
4 concentration was further increased, the removal rate reached a plateau. Additionally, the removal rate of Sb(V) with PFS coagulation decreased from 94% to 51% with the presence of 1 mg·L
−1 KMnO
4 and further decreased to 29% with the presence of 10 mg·L
−1 KMnO
4.
Figure 5b shows that when the aeration time was increased from 0 min to 40 min, the removal rate of Sb(III) decreased from 94% to 79%. However, when the aeration time was further increased, the reduction in the Sb(III) removal rate was negligible. The removal rate of Sb(V) also decreased by 36% with aeration for 60 min. Besides the oxidation role of aeration, the decrease in Sb removal rate was ascribed to formed bubbles having affected the characteristics and affinity of the flocs, thereby inhibiting the adsorption of Sb onto the flocs [
25].
3.6. Mechanisms
There were many possible mechanisms for the removal of Sb(III) and Sb(V) with iron-based coagulants, such as adsorption, precipitation, and coprecipitation. However, previous studies have demonstrated that precipitation and coprecipitation were negligible, which was in accord with our results [
18,
19]. Thereby, we attributed the removal of Sb by coagulation to the adsorption of HFO flocs, and we analyzed the mechanism and function of adsorption onto formed HFO flocs in the removal of Sb.
The combination of in situ formed HFO and Sb(III) or Sb(V) was investigated via FTIR spectra analysis (
Figure 6). The spectral bands that appeared at around 3509 and 1641 cm
−1 were attributed to the stretching and bending vibrations of -OH groups, respectively, for the adsorption of water on the surface of precipitates [
31]. Peaks at 1402 cm
−1 were ascribed to the bending vibrations of -OH groups connected to Sb and iron [
32]. Peaks near 646 cm
−1 were assigned to the Fe–OH bonds of precipitates, and the small shift of those bands was attributed to the adsorption of Sb(III) and Sb(V) [
33]. The small peaks at 590 and 487 cm
−1 of HFO-Sb(V), which were observed for the precipitates produced by coagulation of Sb(V) with PFS and FC, were due to the symmetric stretching and bending vibrations of Sb(V)–O bonds, respectively [
32]. In addition, the Sb(V)–O bonds did not appear in the spectrum of precipitates produced by the coagulation of Sb(III), which indicates that no oxidation of Sb(III) appeared on the surface of iron hydrolytic precipitates.
The in situ formed HFO by iron-based coagulation was highly active, but it was also unstable and easy to turn into ferric hydroxide precipitation of low activity. PFS, with the molecular formula of (Fe2(OH)n(SO4)3-n/2)m, was much more complex than FC (FeCl3). The hydrolyzed and polymerized iron ions in the aqueous solution formed more stable HFO flocs, and the addition of hydroxyl content facilitated the chemical bonding between HFO flocs and Sb. In the coagulation process with FeSO4, flocs formed by the hydrolysis of Fe2+ ions were simple mononuclear complex, and their adsorption capacity was poorer than that of flocs formed by the hydrolysis of PFS and FC. In addition, the electrostatic interaction between the positive HFO flocs and negative Sb(OH)6− facilitated the removal of Sb(V).
4. Conclusions
The performance of Fe(III)-based (PFS, FC) and Fe(II)-based (FeSO4) coagulants on the removal of Sb(III) and Sb(V) was investigated. The removal efficiency of Fe(III)-based coagulants (PFS and FC) was higher than that of the Fe(II)-based coagulant (FeSO4). At the optimum pH of 5, by using Fe(III)-based salts with coagulant dose of 10 mg·L−1 at the initial Sb loading of 200 μg·L−1, the removal rate of Sb(III) (97% with PFS and 98% with FC) was higher than that of Sb(V) (91% with PFS and 93% with FC), but the opposite was true with Fe(II)-based salts, wherein 61% of Sb(III) and 76% of Sb(V) were removed. The adsorption capacities by using PFS, FC, and FeSO4 fit the Sips adsorption isotherms well, and the simulated maximum adsorption capacities were 350 mg·g−1 for Sb(III) and 264 mg·g−1 for Sb(V) by coagulation with PFS. The results indicated that the removal of Sb was attributed to the heterogenous adsorption of HFO at low initial Sb loadings, which was supported by the results of FTIR spectrum analysis. Oxidation inhibited the removal of Sb(III) remarkably. KMnO4, with its strong oxidizing property, remarkably decreased the removal efficiency of Sb. Moreover, the aeration process reduced the removal rate of Sb. In practical applications, Fe(III)-based coagulants have great potential in the treatment of Sb contaminations in rivers or lakes due to their effective and efficient performance, as well as advantages of conveniences and low cost. Furthermore, the oxidation process could be avoided in the removal of Sb(III).