Distribution Coefficient and Metal Pollution Index in Water and Sediments: Proposal of a New Index for Ecological Risk Assessment of Metals
Abstract
:1. Introduction
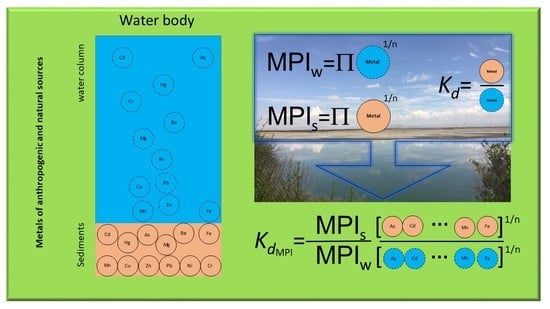
1.1. Metal Pollution Index
1.2. Distribution Coefficient
1.3. Saline Lakes
2. Materials and Methods
2.1. Study Area
2.2. Analytical Procedure for Metals
2.3. Index Assessment
2.4. Statistical Analysis
3. Results
3.1. Chemical Characteristics of Lakes
3.2. Metal Pollution Index
3.3. Distribution Coefficient
3.4. ANOVA and Hierarchical Cluster Analysis
3.5. Discriminant Analysis
3.6. Mean Distribution Coefficient: Ratio MPIS/MPIW as a New Index.
4. Discussion
4.1. Comparison of Metal Concentrations
4.2. Metal Pollution Index
4.3. Distribution Coefficient
4.4. Mean Distribution Coefficient
5. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Tang, W.; Shan, B.; Zhang, W.; Zhang, H.; Wang, L.; Ding, Y. Metal pollution characteristics of surface sediments in different aquatic ecosystems in Eastern China: A Comprehensive understanding. PLoS ONE 2014, 9, 1–7. [Google Scholar] [CrossRef]
- Al-Obaidy, A.H.M.J.; Al-Janabi, Z.Z.; Al-Mashhady, A.A.M. Distribution of some metals in sediments and water in Tigris River. J. Global Ecol. Environ. 2016, 4, 140–146. [Google Scholar]
- Fernández-Buces, N.; Siebe, C.; Cram, S.; Palacio, J.L. Mapping soil salinity using a combined spectral response index for bare soil and vegetation: A case study in the former lake Texcoco, Mexico. J. Arid Environ. 2006, 65, 644–667. [Google Scholar] [CrossRef]
- Alkhatib, E.A.; Grunzke, D.; Chabot, T. Multi-Regression Prediction of Metal Partition Coefficients under Various Physical/Chemical Conditions Design of Experiments As, Cr, Cu, Ni and Zn. Hydrol. Curr. Res. 2016, 7, 1–7. [Google Scholar]
- Singovszka, E.; Balintova, M.; Demcak, S.; Pavlikova, P. Metal Pollution Indices of Bottom Sediment and Surface Water Affected by Acid Mine Drainage. Metals 2017, 7, 284. [Google Scholar] [CrossRef]
- Wurtsbaugh, W.A.; Miller, C.; Null, S.E.; DeRose, J.; Wilcock, P.; Hahnenberger, M.; Howe, F.; Moore, J. Decline of the world’s saline lakes. Nat. Geosci. 2017, 10, 816–821. [Google Scholar] [CrossRef]
- Thorne, R.S.J.; Williams, W.P. The response of benthic macroinvertebrates to pollution in developing countries: A multimetric system of bioassessment. Freshw. Biol. 1997, 37, 671–686. [Google Scholar] [CrossRef]
- Şener, S.; Davraz, A.; Karagüzel, R. Assessment of trace metal contents in water and bottom sediments from Egirdir Lake, Turkey. Environ. Earth Sci. 2014, 71, 2807–2819. [Google Scholar]
- Gurung, B.; Kominkova, D.; Race, M.; Fabricino, M. Assessment of toxic metals mobility in water-sediment environment of the Lambro Creek, Italy. In Proceedings of the 15th International Conference on Environmental Science and Technology, Rhodes, Greece, 31 August–2 September 2017; CEST: Athens, Greece, 2017. [Google Scholar]
- Njenga, J.W.; Ramanathan, A.L.; Subramanian, V. Partitioning of metals in the sediments of Lake Naivasha, Kenya. Chem. Spec. Bioavailab. 2009, 21, 41–48. [Google Scholar] [CrossRef]
- Rajeshkumar, S.; Liu, Y.; Zhang, X.; Ravikumar, B.; Bai, G.; Li, X. Studies on seasonal pollution of metals in water, sediment, fish and oyster from the Meiliang Bay of Taihu Lake in China. Chemosphere 2018, 191, 626–638. [Google Scholar] [CrossRef]
- Wu, B.; Wang, G.; Wu, J.; Fu, Q.; Liu, C. Sources of Metals in Surface Sediments and an Ecological Risk Assessment from Two Adjacent Plateau Reservoirs. PLoS ONE 2014, 9, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Angeler, D.G.; Allen, C.R.; Birgé, H.E.; Drakare, S.; McKie, B.G.; Johnson, R.K. Assessing and managing freshwater ecosystems vulnerable to environmental change. Ambio 2014, 43, 113–125. [Google Scholar] [CrossRef] [Green Version]
- Forghani, G.; Farid, M.; Afshin, Q. The Concentration and Partitioning of Metals in Surface Sediments of the Maharlu Lake, SW Iran. Soil Sediment Contam. 2012, 21, 872–888. [Google Scholar] [CrossRef]
- Herojeet, R.; Rishi, M.S.; Kishore, N. Integrated approach of metal pollution indices and complexity quantification using chemometric models in the Sirsa Basin, Nalagarh valley, Himachal Pradesh, India. Chin. J. Geochem. 2015, 34, 620–633. [Google Scholar] [CrossRef]
- Giancoli Barreto, S.R.; Barreto, W.J.; Deduch, E.M. Determination of partition coefficients of metals in natural tropical water. Clean Soil Air Water 2011, 39, 362–367. [Google Scholar] [CrossRef]
- Boyer, P.; Wells, C.; Howard, B. Extended Kd distributions for freshwater environment. J. Environ. Radioactiv. 2018, 192, 128–142. [Google Scholar] [CrossRef]
- Usero, J.; González, E.; Regalado, L.; Gracia, I. Trace Metals in the Bivalve Mollusc Chamelea gallina from the Atlantic Coast of Southern Spain. Baseline 1996, 32, 305–310. [Google Scholar] [CrossRef]
- Sedeño-Díaz, J.E.; Rodríguez-Romero, A.J.; Mendoza-Martínez, E.; López-López, E. Chemometric Analysis of Wetlands Remnants of the Former Texcoco Lake: A Multivariate Approach. In Lake Sciences and Climate Change, 1st ed.; Mohamed, N.R., Ed.; Intech Open: Rijeca, Croatia, 2016; Volume 8, pp. 135–153. [Google Scholar]
- Takata, H.; Keiko, T.; Tatsuo, A.; Shigeo, U. Distribution coefficients (Kd) of strontium and significance of oxides and organic matter in controlling its partitioning in coastal regions of Japan. Sci. Total Environ. 2014, 490, 979–986. [Google Scholar] [CrossRef]
- Thanh-Nho, N.; Stardy, E.; Nhu-Trang, T.T.; David, F.; Marchand, C. Trace metals partitioning between particulate and dissolved phases along a tropical mangrove estuary (Can Gio, Vietnam). Chemosphere 2018, 196, 311–322. [Google Scholar] [CrossRef]
- Nabelkova, J.; Kominkova, D. Trace Metals in the Bed Sediment of Small Urban Streams. Open Environ. Biol. Monit. J. 2012, 5, 48–55. [Google Scholar] [CrossRef] [Green Version]
- Durrieu, G.; Ciffroy, P.; Garnier, J.M. A weighted bootstrap method for the determination of probability density functions of freshwater distribution coefficients (Kds) of Co, Cs, Sr and I radioisotopes. Chemosphere 2006, 65, 1308–1320. [Google Scholar] [CrossRef]
- Taher, A.; Soliman, A. Metal concentrations in surficial sediments from Wadi El Natrun saline lakes, Egypt. Inter. J. Salt Lake Res. 1999, 8, 75–92. [Google Scholar] [CrossRef]
- Forghani, G.; Moore, F.; Lee, S.; Qishlaqi, A. Geochemistry and speciation of metals in sediments of the Maharlu Saline Lake, Shiraz, SW Iran. Environ. Earth Sci. 2009, 59, 173–184. [Google Scholar] [CrossRef]
- Alcocer, J.; Williams, W.D. Historical and recent changes in Lake Texcoco, a saline lake in Mexico. J. Salt Lake Res. 1996, 5, 45–61. [Google Scholar] [CrossRef]
- Luna-Guido, M.L.; Beltrán-Hernández, R.I.; Solís-Ceballos, N.A.; Hernández-Chávez, N.; Mercado-García, F.; Catt, J.A.; Olalde-Portugal, V.; Dendooven, L. Chemical and biological characteristics of alkaline saline soils from the former Lake Texcoco as affected by artificial drainage. Biol. Fertil. Soils 2000, 32, 102–108. [Google Scholar] [CrossRef]
- Navas, A.; Lindhorfer, H. Geochemical speciation of metals in semiarid soils of the central Ebro Valley (Spain). Environ. Inter. 2003, 29, 61–68. [Google Scholar] [CrossRef] [Green Version]
- Leal, J.A.R.; Medrano, C.N.; Silva, F.T. Aquifer vulnerability and groundwater quality in mega cities: Case of the Mexico Basin. Environ. Earth Sci. 2010, 61, 1309–1320. [Google Scholar] [CrossRef]
- Arce, J.L.; Layer, P.W.; Macías, J.L.; Morales-Casique, E.; García-Palomo, A.; Jiménez-Domínguez, F.J.; Benowitz, J.; Vásquez-Serrano, A. Geology and stratigraphy of the Mexico Basin (Mexico City), central Trans-Mexican Volcanic Belt. J. Maps 2019, 15, 320–332. [Google Scholar] [CrossRef]
- Lorenzo, J.L.; Mirambell, L. Tlapacoya: 35000 años de historia del Lago de Chalco. Colección Científica INAH-SEP, Serie Prehistoria; Instituto Nacional de Antropología e Historia: Mexico City, México, 1986; p. 297.
- Elizondo, R.R. Memoria de las Obras del Sistema de Drenaje Profundo del Distrito Federal; Departamento del Distrito Federal: Ciudad de México, México, 1975. [Google Scholar]
- Alcantara, J.L.; Escalante, P. Current Threats to the Lake Texcoco Globally Important Bird Area; USDA Forest Service: Washington, DC, USA, 2005.
- García, E. Modificaciones al Sistema de Clasificación Climática (Para Adaptarlo a las Condiciones de la República Mexicana, 6th ed.; UNAM: Mexico City, Mexico, 2004; p. 90. [Google Scholar]
- Government of Mexico. Available online: https://www.gob.mx/cms/uploads/attachment/file/166769/NMX-AA-014-1980.pdf (accessed on 18 September 2019).
- USEPA. Methods for Collection, Storage and Manipulation of Sediments for Chemical and Toxicological Analyses: Technical Manual; EPA-823-B-01-002; United States Environmental Protection Agency: Washington, DC, USA, 2001.
- Ministry of Economy. Available online: http://www.economia-nmx.gob.mx/normas/nmx/2010/nmx-aa-132-scfi-2016.pdf (accessed on 18 September 2019).
- Government of Mexico. Available online: https://www.gob.mx/cms/uploads/attachment/file/166150/nmx-aa-115-scfi-2015.pdf (accessed on 18 September 2019).
- Government of Mexico. Available online: https://www.gob.mx/cms/uploads/attachment/file/ 166788/NMX-AA-072-SCFI-2001.pdf (accessed on 8 December 2019).
- Government of Mexico. Available online: https://www.gob.mx/cms/uploads/attachment/file/ 166146/nmx-aa-034-scfi-2015.pdf (accessed on 8 December 2019).
- Navarrete-López, M.; Jonathan, M.P.; Rodríguez-Espinosa, P.F.; Salgado-Galeana, J.A. Autoclave decomposition method for metals in soils and sediments. J. Environ. Monit. Assess. 2012, 184, 2285–2293. [Google Scholar] [CrossRef]
- Government of Mexico. Available online: https://www.gob.mx/cms/uploads/attachment/file/166785/NMX-AA-051-SCFI-2001.pdf (accessed on 18 September 2019).
- Martin, T.D.; Brockhoff, C.A.; Creed, J.T. EMMC Methods Work Group—Method 200.7, Revision 4.4: Determination of Metals and Trace Elements in Water and Wastes by Inductively Coupled Plasma-Atomic Emission Spectrometry; Environmental Protection Agency: Cincinnati, OH, USA, 1994; p. 59.
- SGS-U.S. Geological Survey Office of Water Quality. USGS Water-Quality Information: Water Hardness and Alkalinity. Available online: https://canvas.jmu.edu>files>dowload (accessed on 18 September 2019).
- Bahnasawy, M.; Khidr, A.A.; Dheina, N. Assessment of heavy metal concentrations in water, plankton, and fish of Lake Manzala, Egypt. Turk. J. Zool. 2011, 35, 271–280. [Google Scholar] [CrossRef] [Green Version]
- Moore, F.; Forghan, G.; Qishlaq, A. Assessment of heavy metal contamination in water and surface sediments of the Maharlu saline lake, SW Iran. Iran J. Sci. Technol. 2009, 33, 43–55. [Google Scholar]
- Ochieng, E.Z.; Lalah, J.O.; Wandiga, S.O. Analysis of Heavy Metals in Water and Surface Sediment in Five Rift Valley Lakes in Kenya for Assessment of Recent Increase in Anthropogenic Activities. Bull. Environ. Contam. Toxicol. 2007, 79, 570–576. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Li, W. Dissolved trace elements and heavy metals from the shallow lakes in the middle and lower reaches of the Yangtze River region, China. Environ. Earth Sci. 2011, 62, 1503–1511. [Google Scholar] [CrossRef]
- Stojanovica, A.; Kogelniga, D.; Mittereggera, B.; Maderb, D.; Jirsaa, F.; Krachlera, R.; Krachler, R. Major and trace element geochemistry of superficial sediments and suspended particulate matter of shallow saline lakes in Eastern Austria. Chem. Erde-Geochem. 2009, 69, 223–234. [Google Scholar] [CrossRef]
- Bayanmunkh, B.; Sen-Lin, T.; Narangarvuu, D.; Ochirkhuyag, B.; Bolormaa, O. Physico-Chemical Composition of Saline Lakes of the Gobi Desert Region, Western Mongolia. J. Earth Sci. Clim. Chang. 2017, 8, 1–7. [Google Scholar]
- Obluchinskaya, E.D.; Aleshina, E.G.; Matishov, D.G. Comparative Assessment of the Metal Load in the Bays and Inlets of Murmansk Coast by the Metal Pollution Index. Oceanology 2013, 448, 236–239. [Google Scholar] [CrossRef]
- Öğlü, B.; Yorulmaz, B.; Genç, T.O.; Yilmaz, F. The Assessment of Metal Content by Using Bioaccumulation Indices in European Chub, Squalius cephalus (Linnaeus, 1758). Carpath. J. Earth Environ. 2015, 10, 85–94. [Google Scholar]
- Dadolahi, A.S.; Nazarizadeh, M.D. Metals Contamination in Sediments from the North of the Strait of Hormuz. Mar. Sci. 2013, 4, 39–46. [Google Scholar]
- Adeniyi, A.A.; Owoade, O.J.; Shotonwa, I.O.; Okedeyi, O.O.; Ajibade, A.A.; Sallu, A.R.; Olawore, M.A.; Ope, K.A. Monitoring metals pollution using water and sediments collected from Ebute Ogbo river catchments, Ojo, Lagos, Nigeria. Afr. J. Pure Appl. Chem. 2011, 5, 219–223. [Google Scholar]
- Li, Y.; Liu, F.; Zhou, X.; Wnag, X.; Liu, Q.; Zhu, P.; Zhang, L.; Sun, C. Distribution and Ecological Risk Assessment of Metals in Sediments in Chinese Collapsed Lakes. Pol. J. Environ. Stud. 2017, 26, 181–188. [Google Scholar] [CrossRef]
- Allison, J.D.; Allison, T.L. Partition Coefficients for Metals in Surface Water, Soil, and Waste; U.S. Environmental Protection Agency: Washington, DC, USA, 2005; p. 93.
- Turner, A. Trace-metal partitioning in estuaries: Importance of salinity and particle concentration. Mar. Chem. 1996, 54, 27–39. [Google Scholar] [CrossRef]
- Baeyens, W.; Parmentier, K.; Goeyens, L.; Ducastel, G.; De Gieter, M.; Leermakers, M. The biogeochemical behaviour of Cd, Cu, Pb and Zn in the Scheldt estuary: Results of the 1995 surveys. Hydribiología 1998, 366, 45–62. [Google Scholar] [CrossRef]
- Seida, Y. Influence of Salinity and pH of Solution on Partition Coefficient of Ion at Solution—Charged Media Interface. J. Toyo Univ. Nat. Sci. 2014, 58, 57–66. [Google Scholar]
- Navas, A.; Machín, J. Spatial distribution of metals and arsenic in soils of Aragón (northeast Spain): Controlling factors and environmental implications. Appl. Geochem. 2002, 17, 961–973. [Google Scholar] [CrossRef] [Green Version]
- Jesus, T. Ecological, anatomical and physiological traits of benthic macroinvertebrates: Their use on the health characterization of freshwater ecosystems. Limnetica 2008, 27, 79–92. [Google Scholar]










| Water Body | Conductivity (µS/cm) | Hardness (mg/L as CaCO3) | TDS (mg/L) | |||
|---|---|---|---|---|---|---|
| ±SE | ±SE | ±SE | ||||
| Churubusco | 2036 | 187 | 237 | 8 | 3705 | 1387 |
| Regulación Horaria | 4882 | 547 | 226 | 11 | 2767 | 372 |
| Fusible | 6397 | 1291 | 214 | 33 | 6939 | 2616 |
| Recreativo | 19,660 | 340 | 240 | 22 | 42,594 | 8037 |
| Nabor Carrillo | 6654 | 493 | 245 | 22 | 5088 | 1016 |
| Water Body | Water (µg/L) | |||||||||||
| As | Ba | Cd | Cu | Cr | Fe | Mg | Mn | Hg | Ni | Pb | Zn | |
| Churubusco | 1.81 | 239 | 9 | 21 | 40 | 717 | 29,029 | 130 | 0.99 | 26 | 75 | 41 |
| Regulación Horaria | 2.13 | 234 | 9 | 16 | 46 | 866 | 27,854 | 57 | 1.24 | 46 | 97 | 41 |
| Fusible | 9.64 | 251 | 17 | 34 | 45 | 1212 | 31,176 | 89 | 1.10 | 100 | 112 | 62 |
| Recreativo | 7.14 | 319 | 80 | 61 | 69 | 639 | 47,469 | 80 | 1.37 | 381 | 458 | 65 |
| Nabor Carrillo | 1.85 | 226 | 9 | 15 | 44 | 353 | 40,353 | 28 | 1.20 | 34 | 90 | 39 |
| Manzala Lake | 20 | 55 | 22 | 311 | ||||||||
| Maharlu Lake | 0.28 | 0.28 | 10,400 | 313,200 | 1500 | 2.36 | 5 | 370 | ||||
| Rift Valley Lakes | 17 | 33 | 85 | 161 | 145 | 171 | 86 | |||||
| Yangtze Lakes | 26 | 81 | 0.90 | 11 | 39 | 0.32 | 1.30 | 24 | 3 | |||
| Water Body | Sediments (ppm) | |||||||||||
| As | Ba | Cd | Cu | Cr | Fe | Mg | Mn | Hg | Ni | Pb | Zn | |
| Churubusco | 0.51 | 122 | 2.26 | 210 | 104 | 12,833 | 13,862 | 551 | 2.15 | 45 | 56 | 356 |
| Regulación Horaria | 0.37 | 174 | 1.61 | 25 | 22 | 11,789 | 38,959 | 329 | 0.44 | 30 | 47 | 86 |
| Fusible | 0.42 | 140 | 2.28 | 57 | 41 | 15,162 | 42,112 | 538 | 1.02 | 38 | 70 | 139 |
| Recreativo | 0.47 | 198 | 1.89 | 108 | 67 | 18,578 | 23,275 | 580 | 1.08 | 42 | 61 | 236 |
| Nabor Carrillo | 0.55 | 144 | 1.66 | 29 | 26 | 13,777 | 27,055 | 423 | 0.64 | 34 | 48 | 86 |
| Maharlu Lake | 0.52 | 3 | 38 | 40 | 19,477 | 554 | 207 | 160 | 67 | |||
| Rift Valley Lakes | 0.64 | 10 | 3 | 1320 | 25 | 20 | 169 | |||||
| Yangtze Lakes | 31 | 583 | 465 | 65 | 837 | 0.03 | 33 | 46 | 144 | |||
| Wadi El Natrum | 27 | 45 | 377 | 26 | 52 | 45 | ||||||
| Austria Lakes | 3 | 141 | 32 | 1.18 | 44 | 70 | ||||||
| Mongolian lakes | 13 | 25 | 243 | 696 | 151 | 16 | 65 | |||||
| Churubusco | Regulación Horaria | Fusible | Recreativo | ||
|---|---|---|---|---|---|
| Kd Fe | Churubusco | ||||
| Regulación Horaria | |||||
| Fusible | |||||
| Recreativo | |||||
| Nabor Carrillo | 0.001 | ||||
| Kd Mn | Churubusco | ||||
| Regulación Horaria | <0.004 | ||||
| Fusible | |||||
| Recreativo | <0.0005 | ||||
| Nabor Carrillo | <0.0001 | 0.003 | |||
| Kd Zn | Churubusco | ||||
| Regulación Horaria | <0.001 | ||||
| Fusible | |||||
| Recreativo | |||||
| Nabor Carrillo | <0.005 | ||||
| Kd Cu | Churubusco | ||||
| Regulación Horaria | <0.001 | ||||
| Fusible | |||||
| Recreativo | |||||
| Nabor Carrillo | |||||
| Kd Mg | Churubusco | ||||
| Regulación Horaria | <0.0001 | ||||
| Fusible | <0.0001 | ||||
| Recreativo | <0.0001 | <0.0001 | |||
| Nabor Carrillo | 0.004 | ||||
| Kd Cr | Churubusco | ||||
| Regulación Horaria | <0.0002 | ||||
| Fusible | |||||
| Recreativo | |||||
| Nabor Carrillo | <0.001 | ||||
| Kd Ni | Churubusco | ||||
| Regulación Horaria | |||||
| Fusible | |||||
| Recreativo | <0.0001 | 0.002 | <0.0005 | ||
| Nabor Carrillo | <0.0001 | ||||
| Kd Pb | Churubusco | ||||
| Regulación Horaria | |||||
| Fusible | <0.001 | ||||
| Recreativo | |||||
| Nabor Carrillo | <0.004 | ||||
| Kd As | Churubusco | ||||
| Regulación Horaria | |||||
| Fusible | |||||
| Recreativo | <0.0001 | ||||
| Nabor Carrillo | <0.0001 | ||||
| Kd Cd | Churubusco | ||||
| Regulación Horaria | |||||
| Fusible | |||||
| Recreativo | <0.0001 | < 0.002 | < 0.0001 | ||
| Nabor Carrillo | <0.0005 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sedeño-Díaz, J.E.; López-López, E.; Mendoza-Martínez, E.; Rodríguez-Romero, A.J.; Morales-García, S.S. Distribution Coefficient and Metal Pollution Index in Water and Sediments: Proposal of a New Index for Ecological Risk Assessment of Metals. Water 2020, 12, 29. https://doi.org/10.3390/w12010029
Sedeño-Díaz JE, López-López E, Mendoza-Martínez E, Rodríguez-Romero AJ, Morales-García SS. Distribution Coefficient and Metal Pollution Index in Water and Sediments: Proposal of a New Index for Ecological Risk Assessment of Metals. Water. 2020; 12(1):29. https://doi.org/10.3390/w12010029
Chicago/Turabian StyleSedeño-Díaz, Jacinto Elías, Eugenia López-López, Erick Mendoza-Martínez, Alexis Joseph Rodríguez-Romero, and Sandra Soledad Morales-García. 2020. "Distribution Coefficient and Metal Pollution Index in Water and Sediments: Proposal of a New Index for Ecological Risk Assessment of Metals" Water 12, no. 1: 29. https://doi.org/10.3390/w12010029