Magnetophoretic Harvesting of Nannochloropsis oculata Using Iron Oxide Immobilized Beads
Abstract
:1. Introduction
2. Materials and Methods
2.1. Algae Culture
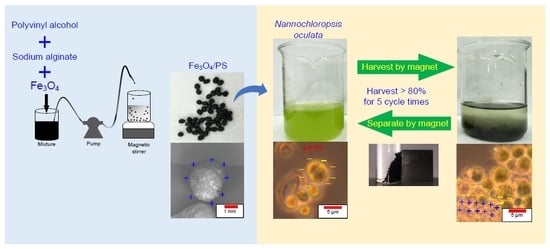
2.2. Fe3O4/PS Preparation
2.3. Characterization Analysis
2.4. Biomass Harvesting
2.5. Biomass Separation and Reusability
2.6. Statistical Analysis
3. Results and Discussion
3.1. Fe3O4/PS Characteristics
3.2. Effects of Dosage Concentration on Magnetic Harvesting
3.3. Effects of pH on Magnetic Harvesting
3.4. Fe3O4/PS Reusability
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Matter, I.A.; Bui, V.K.H.; Jung, M.; Seo, J.Y.; Kim, Y.-E.; Lee, Y.C.; Oh, Y.K. Flocculation harvesting techniques for microalgae: A review. Appl. Sci. 2019, 9, 3069. [Google Scholar] [CrossRef] [Green Version]
- Singh, G.; Patidar, S.K. Microalgae harvesting techniques: A review. J. Environ. Manag. 2018, 217, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Nugroho, Y.K.; Shakeel, S.R.; Li, Z.; Martinkauppi, B.; Hiltunen, E. Using microalgae to produce liquid transportation biodiesel: What is next? Renew. Sustain. Energy Rev. 2017, 78, 391–400. [Google Scholar] [CrossRef]
- Chua, E.T.; Eltanahy, E.; Jung, H.; Uy, M.; Thomas-Hall, S.R.; Schenk, P.M. Efficient harvesting of Nannochloropsis microalgae via optimized chitosan-mediated flocculation. Glob. Chall. 2019, 3, 1800038. [Google Scholar] [CrossRef] [Green Version]
- Yan, C.; Song, X.; Yu, M.; Tong, Y.; Zhang, W. Determining the effects of polyaluminum chloride alkalinities and dosage treatments on various microalgal growth phases for the treatment of microalgae-laden water. Sep. Purif. Technol. 2019, 209, 202–210. [Google Scholar] [CrossRef]
- Surendhiran, D.; Vijay, M. Effect of various pretreatment for extracting intracellular lipid from Nannochloropsis oculata under nitrogen replete and depleted conditions. ISRN Chem. Eng. 2014, 2014, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Sansone, C.; Brunet, C. Promises and challenges of microalgal antioxidant production. Antioxidants 2019, 8, 199. [Google Scholar] [CrossRef] [Green Version]
- Fuad, N.; Omar, R.; Kamarudin, S.; Harun, R.; Idris, A.; Wan, W.A. Mass harvesting of marine microalgae using different techniques. Food Bioprod. Process. 2018, 112, 169–184. [Google Scholar] [CrossRef]
- Han, P.; Lu, Q.; Fan, L.; Zhou, W. A review on the use of microalgae for sustainable aquaculture. Appl. Sci. 2019, 9, 2377. [Google Scholar] [CrossRef] [Green Version]
- Toh, P.Y.; Yeap, S.P.; Kong, L.P.; Ng, B.W.; Chan, D.J.C.; Ahmad, A.L.; Lim, J.K. Magnetophoretic removal of microalgae from fishpond water: Feasibility of high gradient and low gradient magnetic separation. Chem. Eng. J. 2012, 211–212, 22–30. [Google Scholar] [CrossRef]
- Wang, T.; Yang, W.L.; Hong, Y.; Hou, Y.L. Magnetic nanoparticles grafted with amino-riched dendrimer as magnetic flocculant for efficient harvesting of oleaginous microalgae. Chem. Eng. J. 2016, 297, 304–314. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, Y.; Jiang, X.; Liu, L.; Li, X.; Li, H.; Liang, W. In-situ self-assembly of plant polyphenol-coated Fe3O4 particles for oleaginous microalgae harvesting. J. Environ. Manag. 2018, 214, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.D.; Hiltunen, E.; Li, Z. Using magnetic materials to harvest microalgal biomass: Evaluation of harvesting and detachment efficiency. Environ. Technol. 2019, 40, 1006–1012. [Google Scholar] [CrossRef] [PubMed]
- Gao, F. An overview of surface-functionalized magnetic nanoparticles: Preparation and application for wastewater treatment. ChemistrySelect 2019, 4, 6805–6811. [Google Scholar] [CrossRef]
- Gallo-Cordova, A.; Morales, M.P.; Mazarío, E. Effect of the surface charge on the adsorption capacity of chromium (VI) of iron oxide magnetic nanoparticles prepared by microwave-assisted synthesis. Water 2019, 11, 2372. [Google Scholar] [CrossRef] [Green Version]
- Schwaminger, S.P.; Bauer, D.; Fraga-García, P.; Wagner, F.E.; Berensmeier, S. Oxidation of magnetite nanoparticles: Impact on surface and crystal properties. CrystEngComm 2017, 19, 246–255. [Google Scholar] [CrossRef] [Green Version]
- Aruoja, V.; Pokhrel, S.; Sihtmäe, M.; Mortimer, M.; Mädler, L.; Kahru, A. Toxicity of 12 metal-based nanoparticles to algae, bacteria and protozoa. Environ. Sci. Nano 2015, 2, 630–644. [Google Scholar] [CrossRef]
- Vashist, V.; Chauhan, D.; Bhattacharya, A.; Rai, M.P. Role of silica coated magnetic nanoparticle on cell flocculation, lipid extraction and linoleic acid production from Chlorella pyrenoidosa. Nat. Prod. Res. 2019, 11, 1–5. [Google Scholar] [CrossRef]
- Lv, X.; Jiang, G.; Xue, X.; Wu, D.; Sheng, T.; Sun, C.; Xu, X. Fe0-Fe3O4 nanocomposites embedded polyvinyl alcohol/sodium alginate beads for chromium (VI) removal. J. Hazard. Mater. 2013, 262, 748–758. [Google Scholar] [CrossRef]
- Huang, C.F.; Huang, A.C.; Hsieh, Y.F.; Chu, F.J.; Wan, T.J. The effects of magnetic nanoparticles embedded with SA/PVA and pH on chemical-mechanical polishing wastewater and magnetic particle regeneration and recycle. Water Resour. Ind. 2017, 18, 9–16. [Google Scholar] [CrossRef]
- Chen, S.; Hu, J.; Shi, J.; Wang, M.; Guo, Y.; Li, M.; Duo, J.; Deng, T. Composite hydrogel particles encapsulated ammonium molybdophosphate for efficiently cesium selective removal and enrichment from wastewater. J. Hazard. Mater. 2019, 371, 694–704. [Google Scholar] [CrossRef] [PubMed]
- Mahdavinia, G.R.; Etemadi, H. In situ synthesis of magnetic CaraPVA IPN nanocomposite hydrogels and controlled drug release. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 45, 250–260. [Google Scholar] [CrossRef] [PubMed]
- Prabha, G.; Raj, V. Sodium alginate-polyvinyl alcohol-bovin serum albumin coated Fe3O4 nanoparticles as anticancer drug delivery vehicle: Doxorubicin loading and in vitro release study and cytotoxicity to HepG2 and L02 cells. Mater. Sci. Eng. C. Mater. Biol. Appl. 2017, 79, 410–422. [Google Scholar] [CrossRef] [PubMed]
- Matinja, A.I.; Mohd Zain, N.A.; Suhaimi, M.S.; Alhassan, A.J. Optimization of biodiesel production from palm oil mill effluent using lipase immobilized in PVA-alginate-sulfate beads. Renew. Energy. 2019, 135, 1178–1185. [Google Scholar] [CrossRef]
- Chu, F.J.; Wan, T.J.; Pai, T.Y.; Lin, H.W.; Liu, S.H.; Huang, C.F. Use of magnetic fields and nitrate concentration to optimize the growth and lipid yield of Nannochloropsis oculata. J. Environ. Manag. 2020, 253, 109680. [Google Scholar] [CrossRef]
- Taylor, P.; Owen, D.G. Oxidation of Magnetite in Aerated Aqueous Media; Atomic Energy of Canada Ltd.: Pinawa, MB, Canada, 1993. [Google Scholar]
- Yilmaz, G.; Bozkurt, U.; Magden, K.A. Effect of iron ions (Fe2+, Fe3+) on the formation and structure of aerobic granular sludge. Biodegradation 2017, 28, 53–68. [Google Scholar] [CrossRef]
- Hao, W.; Li, Y.; Lv, J.; Chen, L.; Zhu, J. The biological effect of metal ions on the granulation of aerobic granular activated sludge. J. Environ. Sci. 2016, 44, 252–259. [Google Scholar] [CrossRef]
- Ren, X.; Chen, Y.; Guo, L.; She, Z.; Gao, M.; Zhao, Y.; Shao, M. The influence of Fe2+, Fe3+ and magnet powder (Fe3O4) on aerobic granulation and their mechanisms. Ecotoxicol. Environ. Saf. 2018, 164, 1–11. [Google Scholar] [CrossRef]
- Lama, S.; Muylaert, K.; Karki, T.B.; Foubert, I.; Henderson, R.K.; Vandamme, D. Flocculation properties of several microalgae and a cyanobacterium species during ferric chloride, chitosan and alkaline flocculation. Bioresour. Technol. 2016, 220, 464–470. [Google Scholar] [CrossRef] [Green Version]
- Boli, E.; Savvidou, M.; Logothetis, D.; Louli, V.; Pappa, G.; Voutsas, E.; Kolisis, F.; Magoulas, K. Magnetic harvesting of marine algae Nannochloropsis oceanica. Sep. Sci. Technol. 2017, 2017, 1–8. [Google Scholar] [CrossRef]
- Prochazkova, G.; Safarik, I.; Branyik, T. Harvesting microalgae with microwave synthesized magnetic microparticles. Bioresour. Technol. 2013, 130, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Wiśniewska, M.; Ostolska, I.; Szewczuk-Karpisz, K.; Chibowski, S.; Terpilowski, K.; Gun’ko, V.M.; Zarko, V.I. Investigation of the polyvinyl alcohol stabilization mechanism and adsorption properties on the surface of ternary mixed nanooxide AST 50 (Al2O3-SiO2-TiO2). J. Nanopart. Res. 2015, 17, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuad, N.; Omar, R.; Kamarudin, S.; Harun, R.; Idris, A.; Wan Azlina, W.A.K.G. Effective use of tannin based natural biopolymer, AFlok-BP1 to harvest marine microalgae Nannochloropsis. J. Environ. Chem. Eng. 2018, 6, 4318–4328. [Google Scholar] [CrossRef]
- Gerchman, Y.; Vasker, B.; Tavasi, M.; Mishael, Y.; Kinel-Tahan, Y.; Yehoshua, Y. Effective harvesting of microalgae: Comparison of different polymeric flocculants. Bioresour. Technol. 2017, 228, 141–161. [Google Scholar] [CrossRef] [PubMed]
- Abo Markeb, A.; Llimos-Turet, J.; Ferrer, I.; Blanquez, P.; Alonso, A.; Sanchez, A.; Moral-Vico, J.; Font, X. The use of magnetic iron oxide based nanoparticles to improve microalgae harvesting in real wastewater. Water. Res. 2019, 159, 490–500. [Google Scholar] [CrossRef]
- Kavitha, S.; Yukesh Kannah, R.; Rajesh Banu, J.; Kaliappan, S.; Johnson, M. Biological disintegration of microalgae for biomethane recovery-prediction of biodegradability and computation of energy balance. Bioresour. Technol. 2017, 244, 1367–1375. [Google Scholar] [CrossRef]
- Kavitha, S.; Subbulakshmi, P.; Rajesh Banu, J.; Gobi, M.; Tae Yeom, I. Enhancement of biogas production from microalgal biomass through cellulolytic bacterial pretreatment. Bioresour. Technol. 2017, 233, 34–43. [Google Scholar] [CrossRef]





| Condition | Concentration of Soluble Iron Fe2+ (mg/L) | |||
|---|---|---|---|---|
| pH 3 | pH 4 | pH 5 | pH 6 | |
| Fe3O4/PS 1 | 16 ± 0.02 a | 15.9 ± 0.03 a | 15.9 ± 0.03 a | 15.9 ± 0.02 a |
| Naked Fe3O4 2 | 2220 ± 31 d | 1077 ± 67 c | 132 ± 8 b | 22 ± 3 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, F.-J.; Wan, T.-J.; Chen, H.; Wu, C.-H.; Kao, P.-M. Magnetophoretic Harvesting of Nannochloropsis oculata Using Iron Oxide Immobilized Beads. Water 2020, 12, 236. https://doi.org/10.3390/w12010236
Chu F-J, Wan T-J, Chen H, Wu C-H, Kao P-M. Magnetophoretic Harvesting of Nannochloropsis oculata Using Iron Oxide Immobilized Beads. Water. 2020; 12(1):236. https://doi.org/10.3390/w12010236
Chicago/Turabian StyleChu, Feng-Jen, Terng-Jou Wan, His Chen, Chih-Hung Wu, and Po-Min Kao. 2020. "Magnetophoretic Harvesting of Nannochloropsis oculata Using Iron Oxide Immobilized Beads" Water 12, no. 1: 236. https://doi.org/10.3390/w12010236