Optimization of Wastewater Phosphorus Removal in Winter Temperatures Using an Anaerobic–Critical Aerobic Strategy in a Pilot-Scale Sequencing Batch Reactor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Wastewater and Seed Sludge
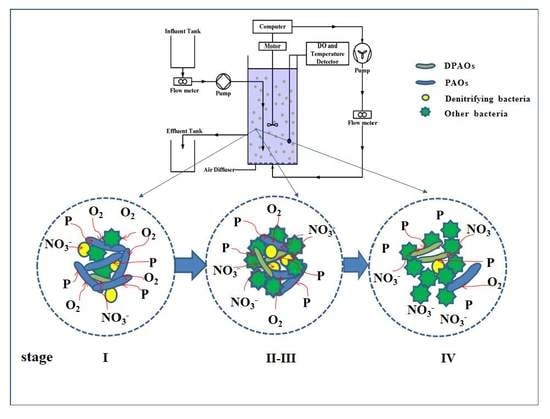
2.2. Reactor Operation
2.3. Physicochemical Analysis
2.4. Morphological Observation
2.5. Microbial Community Analysis
3. Results
3.1. Performances of SBR
3.1.1. Phosphorus Removals
3.1.2. Nitrogen Removals
3.1.3. COD Removals
3.2. Sludge Structure and Microbial Community Composition
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Li, J.; Jin, Y.; Guo, Y.; He, J. Enhancement of phosphorus removal in a low temperature A(2)/O process by anaerobic phosphorus release of activated sludge. Water Sci. Technol. 2013, 67, 2437–2443. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Ren, N.Q.; Wang, X.H.; Kang, H. Effect of temperature on intracellular phosphorus absorption and extra-cellular phosphorus removal in EBPR process. Bioresour. Technol. 2010, 101, 6265–6268. [Google Scholar] [CrossRef] [PubMed]
- Li, X.M.; Chen, H.B.; Yang, Q.; Wang, D.B.; Luo, K.; Zeng, G.M. Biological nutrient removal in a sequencing batch reactor operated as oxic/anoxic/extended-idle regime. Chemosphere 2014, 105, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Oehmen, A.; Lemos, P.C.; Carvalho, G.; Yuan, Z.; Kellerb, J.; Blackall, L.L.; Reis, M.A.M. Advances in enhanced biological phosphorus removal: From micro to macro scale. Water Res. 2007, 41, 2271–2300. [Google Scholar] [CrossRef]
- Mace, S.; Mata-Alvarez, J. Utilization of SBR Technology for Wastewater Treatment: An Overview. Ind. Eng. Chem. Res. 2002, 41, 5539–5553. [Google Scholar] [CrossRef]
- Panswad, T.; Doungchai, A.; Anotaib, J. Temperature effect on microbial community of enhanced biological phosphorus removal system. Water Res. 2003, 37, 409–415. [Google Scholar] [CrossRef]
- Jena, J.; Kumarb, R.; Saifuddina, M.; Dixitb, A.; Dasa, T. Anoxic–aerobic SBR system for nitrate, phosphate and COD removal from high-strength wastewater and diversity study of microbial communities. Biochem. Eng. J. 2016, 105, 80–89. [Google Scholar] [CrossRef]
- Liu, G.; Wang, J. Long-term low DO enriches and shifts nitrifier community in activated sludge. Environ. Sci. Technol. 2013, 47, 5109–5117. [Google Scholar] [CrossRef]
- McCarty, P.L.; Bae, J.; Kim, J. Domestic wastewater treatment as a net energy producer--can this be achieved? Environ. Sci. Technol. 2011, 45, 7100–7106. [Google Scholar] [CrossRef]
- Liu, S.; Li, J. Accumulation and isolation of simultaneous denitrifying polyphosphate-accumulating organisms in an improved sequencing batch reactor system at low temperature. Int. Biodeter. Biodegr. 2015, 100, 140–148. [Google Scholar] [CrossRef]
- Liu, Y.; Li, X.; Kang, X.; Yuan, Y. Performance of denitrifying phosphorus removal of Acinetobacteria strain at low temperature. Int. Biodeter. Biodegr. 2014, 95, 135–138. [Google Scholar] [CrossRef]
- Fan, X.Y.; Gao, J.F.; Pan, K.L.; Li, D.C.; Dai, H.H.; Li, X. Temporal heterogeneity and temperature response of active ammonia-oxidizing microorganisms in winter in full-scale wastewater treatment plants. Chem. Eng. J. 2019, 360, 1542–1552. [Google Scholar] [CrossRef]
- Miao, Z.; Zeng, W.; Wang, S.; Peng, Y.; Cao, G.; Weng, D.; Xue, G.; Yang, Q. Effect of temperature on anoxic metabolism of nitrites to nitrous oxide by polyphosphate accumulating organisms. J. Environ. Sci. 2014, 26, 264–273. [Google Scholar] [CrossRef]
- Zhang, H.L.; Sheng, G.P.; Fang, W.; Wang, Y.P.; Fang, C.Y.; Shao, L.M.; Yu, H.Q. Calcium effect on the metabolic pathway of phosphorus accumulating organisms in enhanced biological phosphorus removal systems. Water Res. 2015, 84, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Gu, S.; Peng, Y.; Wang, S.; Liu, X. Progress in the Development of Control Strategies for the SBR Process. Clean Soil Air Water 2010, 38, 732–749. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater; American Public Health Association (APHA): Washington, DC, USA, 2005. [Google Scholar]
- Liu, R.; Yu, Z.; Guo, H.; Liu, M.; Zhang, H.; Yang, M. Pyrosequencing analysis of eukaryotic and bacterial communities in faucet biofilms. Sci. Total Environ. 2012, 435–436, 124–131. [Google Scholar] [CrossRef]
- Xiong, H.; Zou, D.; Zhou, D.; Dong, S.; Wang, J.; Rittmann, B.E. Enhancing degradation and mineralization of tetracycline using intimately coupled photocatalysis and biodegradation (ICPB). Chem. Eng. J. 2017, 316, 7–14. [Google Scholar] [CrossRef]
- Hu, J.Y.; Ong, S.L.; Ng, W.J.; Lu, F.; Fan, X.J. A new method for characterizing denitrifying phosphorus removal bacteria by using three different types of electron acceptors. Water Res. 2003, 37, 3463–3471. [Google Scholar] [CrossRef]
- Kuba, T.; Van Loosdrecht, M.C.M.; Heijnen, J.J. Phosphorus and nitrogen removal with minimal COD requirement by integration of denitrifying dephosphatation and nitrification in a two-sludge system. Water Res. 1996, 30, 1702–1710. [Google Scholar] [CrossRef]
- Wentzel, M.; Loewenthal, R.; Ekama, G.; Marais, G. Enhanced polyphosphate organism cultures in activated sludge systems-Part 1: Enhanced culture development. Water SA 1988, 15, 71–88. [Google Scholar]
- Chen, J.; Tannahill, A.L.; Shuler, M.L. Design of a System for the Control of Low Dissolved Oxygen Concentrations: Critical Oxygen Concentrations for Azo tobac ter vinelandii and Esch erichia coli. Biotechnol. Bioeng. 1984, 27, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Sayi-Ucar, N.; Sarioglu, M.; Insel, G.; Cokgor, E.U.; Orhon, D.; van Loosdrecht, M.C. Long-term study on the impact of temperature on enhanced biological phosphorus and nitrogen removal in membrane bioreactor. Water Res. 2015, 84, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Zeng, R.J.; Saunder, A.M.S.; Yuan, Z.; Blackall, L.L.; Keller, J. Identification and Comparison of Aerobic and Denitrifying Polyphosphate-Accumulating Organisms. Biotechnol. Bioeng. 2003, 83, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Park, H.D.; Noguera, D.R. Evaluating the effect of dissolved oxygen on ammonia-oxidizing bacterial communities in activated sludge. Water Res. 2004, 38, 3275–3286. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Dai, X.; Chai, X. Effect of different carbon sources on denitrification performance, microbial community structure and denitrification genes. Sci. Total Environ. 2018, 634, 195–204. [Google Scholar] [CrossRef]
- Chu, L.; Wang, J. Denitrification performance and biofilm characteristics using biodegradable polymers PCL as carriers and carbon source. Chemosphere 2013, 91, 1310–1316. [Google Scholar] [CrossRef]
- Hamlin, H.J.; Michaels, J.T.; Beaulaton, C.M.; Graham, W.F.; Dutt, W.; Steinbach, P.; Losordo, T.M.; Schrader, K.K.; Main, K.L. Comparing denitrification rates and carbon sources in commercial scale upflow denitrification biological filters in aquaculture. Aquacult. Eng. 2008, 38, 79–92. [Google Scholar] [CrossRef]
- Srinandan, C.S.; D’Souza, G.; Srivastava, N.; Nayak, B.B.; Nerurkar, A.S. Carbon sources influence the nitrate removal activity, community structure and biofilm architecture. Bioresour. Technol. 2012, 117, 292–299. [Google Scholar] [CrossRef]
- Fan, H.; Liu, X.; Wang, H.; Han, Y.; Qi, L.; Wang, H. Oxygen transfer dynamics and activated sludge floc structure under different sludge retention times at low dissolved oxygen concentrations. Chemosphere 2017, 169, 586–595. [Google Scholar] [CrossRef]
- Zeng, R.J.; Yuan, Z.; Keller, J. Enrichment of denitrifying glycogen-accumulating organisms in anaerobic/anoxic activated sludge system. Biotechnol. Bioeng. 2003, 81, 397–404. [Google Scholar] [CrossRef]
- Carvalho, G.; Lemos, P.C.; Oehmen, A.; Reis, M.A.M. Denitrifying phosphorus removal: Linking the process performance with the microbial community structure. Water Res. 2007, 41, 4383–4396. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Guo, G.; Wang, H.; Stephenson, T.; Guo, J.; Ye, L. Long-term impact of anaerobic reaction time on the performance and granular characteristics of granular denitrifying biological phosphorus removal systems. Water Res. 2013, 47, 5326–5337. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Zhang, X.; Feng, L. Effect of different types of electron acceptors on the anoxic phosphorus uptake activity of denitrifying phosphorus removing bacteria. Bioresour. Technol. 2010, 101, 1603–1610. [Google Scholar] [CrossRef] [PubMed]
- Waki, M.; Yasuda, T.; Fukumoto, Y.; Beline, F.; Magri, A. Treatment of swine wastewater in continuous activated sludge systems under different dissolved oxygen conditions: Reactor operation and evaluation using modelling. Bioresour. Technol. 2017, 250, 574–582. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Qian, F.; Shen, Y.; Liu, X.; Liu, W.; Wang, S. Cultivation and characteristics of partial nitrification granular sludge in a sequencing batch reactor inoculated with heterotrophic granules. Appl. Microbiol. Biot. 2016, 100, 9381–9391. [Google Scholar] [CrossRef] [PubMed]
- Albertsen, M.; Hansen, L.B.S.; Saunders, A.M.; Nielsen, P.H.; Nielsen, K.L. A metagenome of a full-scale microbial community carrying out enhanced biological phosphorus removal. ISME J. 2011, 6, 1094–1106. [Google Scholar] [CrossRef]
- Hanno, T.; Glockner, F.O. Current opportunities and challenges in microbial metagenome analysis-a bioinformatic perspective. Brief. Bioinform. 2012, 13, 728–742. [Google Scholar]
- Crocetti, G.R.; Hugenholtz, P.; Bond, P.L.; Schuler, A.; Keller, J.; Jenkins, D.; Blackall, L.L. Identification of Polyphosphate-Accumulating Organisms and Design of 16S rRNA-Directed Probes for Their Detection and Quantitation. Appl. Environ. Microb. 2000, 66, 1175–1182. [Google Scholar] [CrossRef] [Green Version]
- Hesselmanni, R.P.X.; Werlen, C.; Hahnz, D.; Meer, J.R.V.D.; Zehnder, A.B. Enrichment, Phylogenetic Analysis and Detection of a Bacterium That Performs Enhanced Biological Phosphate Removal in Activated Sludge. System. Appl. Microbiol. 1999, 22, 454–465. [Google Scholar] [CrossRef]
- Carosia, M.F.; Okada, D.Y.; Sakamoto, I.K.; Silva, E.L.; Varesche, M.B.A. Microbial characterization and degradation of linear alkylbenzene sulfonate in an anaerobic reactor treating wastewater containing soap powder. Bioresour. Technol. 2014, 167, 316–323. [Google Scholar] [CrossRef]
- Shu, D.; He, Y.; Yue, H.; Wang, Q. Microbial structures and community functions of anaerobic sludge in six full-scale wastewater treatment plants as revealed by 454 high-throughput pyrosequencing. Bioresour. Technol. 2015, 186, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wei, D.Y.; Li, F.C.; Zhang, Y.W.; Li, R.H. Sulfur-siderite autotrophic denitrification system for simultaneous nitrate and phosphate removal: From feasibility to pilot experiments. Water Res. 2019, 160, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Fu, B.; Liao, X.; Ding, L.; Ren, H. Characterization of microbial community in an aerobic moving bed biofilm reactor applied for simultaneous nitrification and denitrification. World J. Microb. Biot. 2010, 26, 1981–1990. [Google Scholar] [CrossRef]
- Miao, Y.; Liao, R.; Zhang, X.X.; Wang, Y.; Wang, Z.; Shi, P.; Liu, B.; Li, A. Metagenomic insights into Cr(VI) effect on microbial communities and functional genes of an expanded granular sludge bed reactor treating high-nitrate wastewater. Water Res. 2015, 76, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Shinoda, Y.; Sakai, Y.; Uenishi, H.; Uchihashi, Y.; Hiraishi, A.; Yukawa, H.; Yurimoto, H.; Kato, N. Aerobic and Anaerobic Toluene Degradation by a Newly Isolated Denitrifying Bacterium, Thauera sp. Strain DNT-1. Appl. Environ. Microb. 2004, 70, 1385–1392. [Google Scholar] [CrossRef] [Green Version]
- Wen, X.; Zhou, J.; Li, Y.; Qing, X.; He, Q. A novel process combining simultaneous partial nitrification, anammox and denitrification (SNAD) with denitrifying phosphorus removal (DPR) to treat sewage. Bioresour. Technol. 2016, 222, 309–316. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, Q.; Zhang, J.; Wang, C.; Wang, S.; Peng, Y. Enhancement of denitrifying phosphorus removal and microbial community of long-term operation in an anaerobic anoxic oxicebiological contact oxidation system. J. Biosci. Bioeng. 2016, 122, 456–466. [Google Scholar] [CrossRef]
- Albertsen, M.; McIlroy, S.J.; Stokholm-Bjerregaard, M.; Karst, S.M.; Nielsen, P.H. “Candidatus Propionivibrio aalborgensis”: A Novel Glycogen Accumulating Organism Abundant in Full-Scale Enhanced Biological Phosphorus Removal Plants. Microbiology 2016, 7, 1–17. [Google Scholar] [CrossRef]
- Lopez-Vazquez, C.M.; Song, Y.I.; Hooijmans, C.M.; Brdjanovic, D.; Moussa, M.S.; Gijzen, H.J.; van Loosdrecht, M.M. Short-term temperature effects on the anaerobic metabolism of glycogen accumulating organisms. Biotechnol. Bioeng. 2007, 97, 483–495. [Google Scholar] [CrossRef]
- Lopez-Vazquez, C.M.; Song, Y.I.; Hooijmans, C.M.; Brdjanovic, D.; Moussa, M.S.; Gijzen, H.J.; van Loosdrecht, M.C. Temperature effects on the aerobic metabolism of glycogen-accumulating organisms. Biotechnol. Bioeng. 2008, 101, 295–306. [Google Scholar] [CrossRef]
- Lopez-Vazquez, C.M.; Oehmen, A.; Hooijmans, C.M.; Brdjanovic, D.; Gijzen, H.J.; Yuan, Z.; van Loosdrecht, M.C. Modeling the PAO-GAO competition: Effects of carbon source, pH and temperature. Water Res. 2009, 43, 450–462. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Qi, L.; Liu, G.; Zhang, Y.; Fan, Q.; Wang, H. Aeration optimization through operation at low dissolved oxygen concentrations: Evaluation of oxygen mass transfer dynamics in different activated sludge systems. J. Environ. Sci. China 2017, 55, 224–235. [Google Scholar] [CrossRef] [PubMed]





| Stage | Operating Term (d) | Operation Mode | Remarks |
|---|---|---|---|
| I | 1–43 | Anaerobic (3 h)–aerobic (5 h) | Accumulation of PAOs. |
| II | 45–64 | Anaerobic (2 h)–critical aerobic (2 h) | Change the aerobic condition to critical aerobic condition; set the length of critical aerobic condition as 2 h. |
| III | 65–94 | Anaerobic (2 h)–critical aerobic (1 h) | Reduce the critical aerobic phase’s time to 1 h. |
| IV | 95–117 | Anaerobic (2 h)–critical aerobic (0.5 h) | Reduce the critical aerobic phase’s time to 0.5 h. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, M.; Yao, B.; Cong, S.; Ma, T.; Zou, D. Optimization of Wastewater Phosphorus Removal in Winter Temperatures Using an Anaerobic–Critical Aerobic Strategy in a Pilot-Scale Sequencing Batch Reactor. Water 2020, 12, 110. https://doi.org/10.3390/w12010110
Liu M, Yao B, Cong S, Ma T, Zou D. Optimization of Wastewater Phosphorus Removal in Winter Temperatures Using an Anaerobic–Critical Aerobic Strategy in a Pilot-Scale Sequencing Batch Reactor. Water. 2020; 12(1):110. https://doi.org/10.3390/w12010110
Chicago/Turabian StyleLiu, Meijun, Bing Yao, Shibo Cong, Taigang Ma, and Donglei Zou. 2020. "Optimization of Wastewater Phosphorus Removal in Winter Temperatures Using an Anaerobic–Critical Aerobic Strategy in a Pilot-Scale Sequencing Batch Reactor" Water 12, no. 1: 110. https://doi.org/10.3390/w12010110