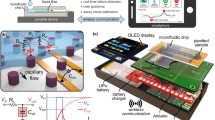
Abstract
Microfluidic devices are well suited for use in field applications, including for point-of-care (POC) diagnosis and therapy monitoring. Advantages of the use of microfluidics include small sample volumes, rapid sample to answer times, and disposable test cards combined with minimal portable instrumentation. A continuing challenge is detection from a complex sample, like saliva, which often requires extensive manual preprocessing to reduce background from interferents and for which analytes are often at lower concentrations than in blood. Further, coupling electrochemical detection to microfluidic devices has shown promise for multiple applications, but most often has been demonstrated with benchtop potentiostats rather than POC-compatible instrumentation. In the current report, we demonstrate a disposable microfluidic flow cell paired with a portable, miniature potentiostat for electrochemical measurement of the anticonvulsant drug carbamazepine in a background of human saliva. Specific highlights of the device include the small input volume of 12 μL of saliva, the absence of any manual preprocessing of the saliva sample, and carbamazepine quantification using an inexpensive polymeric laminate flow cell with stencil-printed electrodes and miniature potentiostat. With this system, accurate and robust quantification of carbamazepine drug level was achieved at therapeutically relevant concentrations of 2.5 μM to 15 μM carbamazepine in saliva. Further, functional dry storage of the microfluidic flow cells was demonstrated over 90 days.
Graphical abstract






Similar content being viewed by others
References
Yager P, Edwards T, Fu E et al (2006) Microfluidic diagnostic technologies for global public health. Nature 442:412–418. https://doi.org/10.1038/nature05064
Olanrewaju A, Beaugrand M, Yafia M, Juncker D (2018) Capillary microfluidics in microchannels: from microfluidic networks to capillaric circuits. Lab Chip 18:2323–2347. https://doi.org/10.1039/C8LC00458G
Byrnes S, Thiessen G, Fu E (2013) Progress in the development of paper-based diagnostics for low-resource point-of-care settings. Bioanalysis 5:2821–2836. https://doi.org/10.4155/bio.13.243
García-Miranda Ferrari A, Rowley-Neale SJ, Banks CE (2021) Screen-printed electrodes: transitioning the laboratory in-to-the field. Talanta Open. https://doi.org/10.1016/j.talo.2021.100032
Stefano JS, Orzari LO, Silva-Neto HA et al (2022) Different approaches for fabrication of low-cost electrochemical sensors. Curr Opin Electrochem 32:100893. https://doi.org/10.1016/j.coelec.2021.100893
Downs C, Nejely A, Fu E (2018) Disposable fabric-based electrochemical sensors fabricated from wax-transfer-printed fluidic cells and stencil-printed electrodes. Anal Methods 10:3696–3703. https://doi.org/10.1039/C8AY01028E
Downs C, Nejely A, Fu E (2019) Integrated wax valve for robust fluid control in an electrochemical fabric-based device. Anal Methods 11:5098–5107. https://doi.org/10.1039/c9ay01648a
Li T, Díaz-Real JA, Holm T (2021) Design of electrochemical microfluidic detectors: a review. Adv Mater Technol 6:2100569. https://doi.org/10.1002/admt.202100569
Mani V, Beduk T, Khushaim W et al (2021) Electrochemical sensors targeting salivary biomarkers: a comprehensive review. TrAC Trends Anal Chem 135:116164
Chiappin S, Antonelli G, Gatti R, De Palo EF (2007) Saliva specimen: a new laboratory tool for diagnostic and basic investigation. Clin Chim Acta 383:30–40. https://doi.org/10.1016/j.cca.2007.04.011
Ngamchuea K, Chaisiwamongkhol K, Batchelor-Mcauley C, Compton RG (2018) Chemical analysis in saliva and the search for salivary biomarkers-a tutorial review. Analyst 143:81–99
Bartlett C-A, Taylor S, Fernandez C et al (2016) Disposable screen printed sensor for the electrochemical detection of methamphetamine in undiluted saliva. Chem Cent J 10:3. https://doi.org/10.1186/s13065-016-0147-2
Ruiz-Valdepenas Montiel V, Sempionatto JR, Esteban-Fernández De Ávila B et al (2018) Delayed sensor activation based on transient coatings: biofouling protection in complex biofluids. J Am Chem Soc 140:14050–14053. https://doi.org/10.1021/jacs.8b08894
Suherman AL, Rasche B, Godlewska B et al (2019) Electrochemical detection and quantification of lithium ions in authentic human saliva using LiMn2O4-modified electrodes. ACS Sens 4:2497–2506. https://doi.org/10.1021/acssensors.9b01176
Rebelo TSCR, Miranda IM, Brandão ATSC et al (2021) A disposable saliva electrochemical MIP-based biosensor for detection of the stress biomarker α-amylase in point-of-care applications. Electrochem 2:427–438. https://doi.org/10.3390/electrochem2030028
Diouf A, Moufid M, Bouyahya D et al (2020) An electrochemical sensor based on chitosan capped with gold nanoparticles combined with a voltammetric electronic tongue for quantitative aspirin detection in human physiological fluids and tablets. Mater Sci Eng C 110:110665. https://doi.org/10.1016/j.msec.2020.110665
Helton KL, Nelson KE, Fu E, Yager P (2008) Conditioning saliva for use in a microfluidic biosensor. Lab Chip 8:1847. https://doi.org/10.1039/b811150b
Wanklyn C, Burton D, Enston E et al (2016) Disposable screen printed sensor for the electrochemical detection of delta-9-tetrahydrocannabinol in undiluted saliva. Chem Cent J 10:1. https://doi.org/10.1186/s13065-016-0148-1
Huang X, Shi W, Li J et al (2020) Determination of salivary uric acid by using poly(3,4-ethylenedioxythipohene) and graphene oxide in a disposable paper-based analytical device. Anal Chim Acta 1103:75–83. https://doi.org/10.1016/J.ACA.2019.12.057
Zack MM, Kobau R (2017) National and state estimates of the numbers of adults and children with active epilepsy—United States, 2015. MMWR Morb Mortal Wkly Rep 66:821–825. https://doi.org/10.15585/mmwr.mm6631a1
Tian N, Croft JB, Kobau R et al (2020) CDC-supported epilepsy surveillance and epidemiologic studies: a review of progress since 1994. Epilepsy Behav 109:107123. https://doi.org/10.1016/j.yebeh.2020.107123
Tian N, Cui W, Zack M et al (2016) Suicide among people with epilepsy: a population-based analysis of data from the U.S. National Violent Death Reporting System, 17 states, 2003–2011. Epilepsy Behav 61:210–217. https://doi.org/10.1016/j.yebeh.2016.05.028
Thurman DJ, Kobau R, Luo Y-H et al (2016) Health-care access among adults with epilepsy: the U.S. National Health Interview Survey, 2010 and 2013. Epilepsy Behav 55:184–188. https://doi.org/10.1016/j.yebeh.2015.10.028
Patsalos PN, Berry DJ, Bourgeois BFD et al (2008) Antiepileptic drugsbest practice guidelines for therapeutic drug monitoring: a position paper by the subcommission on therapeutic drug monitoring, ILAE Commission on Therapeutic Strategies. Epilepsia 49:1239–1276. https://doi.org/10.1111/j.1528-1167.2008.01561.x
Miles MV, Tennison MB, Greenwood RS (1991) Intraindividual variability of carbamazepine, phenobarbital, and phenytoin concentrations in saliva. Ther Drug Monit 13:166–171. https://doi.org/10.1097/00007691-199103000-00013
Rosenthal E, Hoffer E, Ben-Aryeh H et al (1995) Use of saliva in home monitoring of carbamazepine levels. Epilepsia 36:72–74. https://doi.org/10.1111/j.1528-1157.1995.tb01668.x
Patsalos PN, Berry DJ (2013) Therapeutic drug monitoring of antiepileptic drugs by use of saliva. Ther Drug Monit 35:4–29. https://doi.org/10.1097/FTD.0b013e31827c11e7
Ginja Teixeira J, Veiga A, Palace Carvalho AJ, Martins Teixeira D (2013) Electro-oxidation of carbamazepine metabolites: characterization and influence in the voltammetric determination of the parent drug. Electrochim Acta 108:51–65. https://doi.org/10.1016/j.electacta.2013.06.070
Wentland L, Downs C, Fu E (2022) Comparison of signal enhancement strategies for carbamazepine detection in undiluted human saliva using an electrochemical sensor with stencil-printed carbon electrodes. Anal Methods 14:3103–3114. https://doi.org/10.1039/D2AY00926A
Armbruster DA, Pry T (2008) Limit of blank, limit of detection and limit of quantitation. Clin Biochem Rev 29(Suppl 1):S49-52
Downs C, Milovancev M, Fu E (2020) Rational design and characterization of a lateral flow assay for canine C-reactive protein in wound exudate. Talanta 220:121319. https://doi.org/10.1016/j.talanta.2020.121319
Kalanur SS, Seetharamappa J (2010) Electrochemical oxidation of bioactive carbamazepine and its interaction with DNA. Anal Lett 43:618–630. https://doi.org/10.1080/00032710903406870
Wentland L, Polaski R, Fu E (2021) Dry storage of multiple reagent types within a paper microfluidic device for phenylalanine monitoring. Anal Methods 13:660–671. https://doi.org/10.1039/d0ay02043e
Zheng J, Zhou X (2007) Sodium dodecyl sulfate-modified carbon paste electrodes for selective determination of dopamine in the presence of ascorbic acid. Bioelectrochemistry 70:408–415. https://doi.org/10.1016/j.bioelechem.2006.05.011
Cobelo-García A, Santos-Echeandía J, López-Sánchez DE et al (2014) Improving the voltammetric quantification of ill-defined peaks using second derivative signal transformation: example of the determination of platinum in water and sediments. Anal Chem 86:2308–2313. https://doi.org/10.1021/ac403558y
Grushka E, Atamna I (1987) The use of derivatives for establishing integration limits of chromatographic peaks. Chromatographia 24:226–232. https://doi.org/10.1007/BF02688484
Soares DM, Gomes WE, Tenan MA (2007) Sodium dodecyl sulfate adsorbed monolayers on gold electrodes. Langmuir 23:4383–4388. https://doi.org/10.1021/la063508+
Liu L-H, Duan C-Q, Gao Z-N (2012) Electrochemical behaviors and electrochemical determination of carbamazepine at ionic liquid modified carbon paste electrode in the presence of sodium dodecyl sulfate. J Serb Chem Soc 77:483–496. https://doi.org/10.2298/JSC110420188L
Nery EW, Kundys M, Jeleń PS, Jönsson-Niedziólka M (2016) Electrochemical glucose sensing: is there still room for improvement? Anal Chem 88:11271–11282. https://doi.org/10.1021/acs.analchem.6b03151
Sophocleous M, Atkinson JK (2017) A review of screen-printed silver/silver chloride (Ag/AgCl) reference electrodes potentially suitable for environmental potentiometric sensors. Sens Actuators A Phys 267:106–120. https://doi.org/10.1016/J.SNA.2017.10.013
Hughes G, Westmacott K, Honeychurch KC et al (2016) Recent advances in the fabrication and application of screen-printed electrochemical (bio)sensors based on carbon materials for biomedical, agri-food and environmental analyses. Biosensors. https://doi.org/10.3390/bios6040050
Rowe AA, Bonham AJ, White RJ et al (2011) CheapStat: an open-source, “Do-It-Yourself” potentiostat for analytical and educational applications. PLoS ONE 6:e23783. https://doi.org/10.1371/journal.pone.0023783
Dryden MDM, Wheeler AR (2015) DStat: a versatile, open-source potentiostat for electroanalysis and integration. PLoS ONE 10:e0140349. https://doi.org/10.1371/journal.pone.0140349
Gao W, Emaminejad S, Nyein HYY et al (2016) Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature 529:509–514. https://doi.org/10.1038/nature16521
Kim J, Sempionatto JR, Imani S et al (2018) Simultaneous monitoring of sweat and interstitial fluid using a single wearable biosensor platform. Adv Sci 5:1800880. https://doi.org/10.1002/advs.201800880
Song Y, Min J, Yu Y et al (2020) Wireless battery-free wearable sweat sensor powered by human motion. Sci Adv 6:9842. https://doi.org/10.1126/sciadv.aay9842
Tehrani F, Teymourian H, Wuerstle B et al (2022) An integrated wearable microneedle array for the continuous monitoring of multiple biomarkers in interstitial fluid. Nat Biomed Eng. https://doi.org/10.1038/s41551-022-00887-1
Nakamura Y, Kodama H, Satoh T et al (2010) Diurnal changes in salivary amino acid concentrations. In Vivo (Brooklyn) 24:837–842
Neyraud E, Palicki O, Schwartz C et al (2012) Variability of human saliva composition: possible relationships with fat perception and liking. Arch Oral Biol 57:556–566. https://doi.org/10.1016/j.archoralbio.2011.09.016
Acknowledgements
We gratefully acknowledge financial support from OSU and NIH Grant #R21DE031101 (MPIs M.J., S.R., and E.F.). The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
L.W., J.M., and J.C. conducted experiments and analyzed the data. L.W., J.M., and E.F. developed the field-use disposable device. S.R., with support from L.W., J.M., and E.F., developed the analysis algorithm. J.C. and M.J. developed the miniature potentiostat. L.W., J.C., M.J., and E.F. collaborated on platform benchmarking work. L.W. and E.F. wrote the main manuscript text and J.C., M.J., and S.R. contributed key text. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wentland, L., Cook, J.M., Minzlaff, J. et al. Field-use device for the electrochemical quantification of carbamazepine levels in a background of human saliva. J Appl Electrochem 53, 523–534 (2023). https://doi.org/10.1007/s10800-022-01785-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-022-01785-9