Stroke rehab technologies leverage repetition and natural strength to optimize neuroplasticity.
by Kari A. Olk, PT, MSPT, and Shawna Persaud, PhD
PHOTO CAPTION: Marny Farrell, MPT, director of outpatient rehabilitation services, Regions Hospital, demonstrates a powered orthotic device that assists and supports the elbow and shoulder to allow greater independence for people with upper-limb neuromuscular weakness.
Stroke often results in reduced muscle function of the upper and lower extremity, which can dramatically impact an individual’s independence and quality of life. An individual with stroke can suffer persistent changes in their ability to walk and participate in many activities of daily living (ADLs) due to changes in tone and muscle activity that result in gait deviations and decreased functional ability.1-4 Ambulation post stroke is often less efficient and associated with increased energy expenditure.4 Rehabilitation providers play an important role in stroke recovery by helping individuals with improving muscle function after a loss of movement.
The focus of lower extremity stroke rehabilitation is to improve balance, restore range of motion, improve strength, reduce muscle tone and spasticity, and enhance coordination and weight shifting. Since upper extremity stroke rehabilitation focuses on many of the same goals, similar technologies that help to address lower limb impairments can also be applied to upper extremity rehabilitation. In stroke injuries, few patients fully recover from arm weakness after a neurological injury. The remainder of the patients demonstrate persistent arm impairments that are directly linked to activity limitations, participation restrictions, reduced quality of life, and decreased well-being.5
Rehabilitation can help survivors become independent, restore their ability to complete ADLs, and improve quality of life. Even though rehabilitation does not “cure” the effects of stroke in that it does not reverse brain damage, it can substantially help people achieve improved long-term outcomes.6 Recent studies have shown a significant correlation between increased dosage of rehabilitation sessions and improved outcomes.7-9
There is strong consensus among rehabilitation experts that the most important element in any rehabilitation program is carefully directed, well-focused, repetitive practice.10 The repetitive use of impaired limbs encourages brain plasticity and can help reduce the severity of the injury.10 Positive outcomes of physical rehabilitation, in the case of neurologically based disorders, depend heavily on onset, duration, intensity and task-orientation of the training,11 as well as the patient’s health condition, attention and effort.12
A variety of technologies can be used to support rehabilitation goals, including aquatic rehabilitation, body weight support training, functional electrical stimulation, virtual reality, and robotics for use in-clinic and at in-home.
Aquatic Rehabilitation
Aquatic rehabilitation offers a whole-body approach that uses the properties of water and provides body weight support with varying resistance. Additionally, aquatic rehabilitation uses hydrostatic pressure to reduce swelling and help with proprioceptive awareness while reducing impact on the joints. Integration of aquatic therapy has been effective in improving static and dynamic balance as well as enhancing functional capacity in those with acquired brain injury.13 A benefit of aquatic rehabilitation is that it can be done in the clinic, at home or in the community depending on resources.
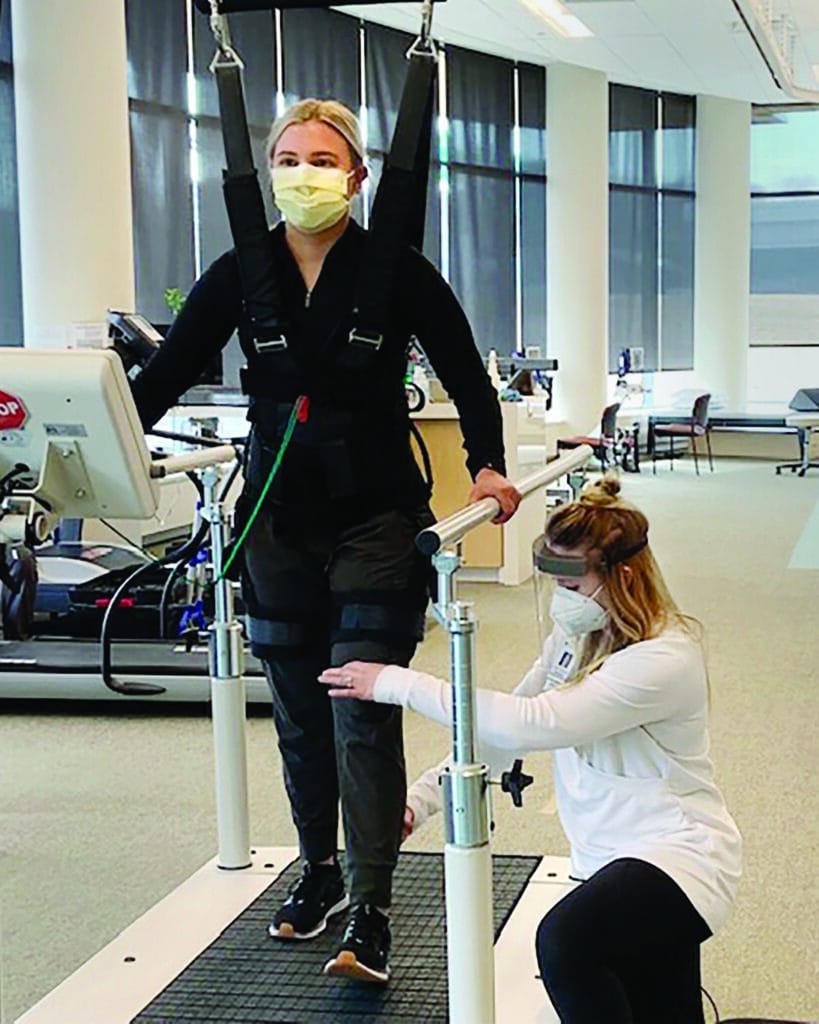
Body Weight Support Training (BWS)
BWS training has been shown to elicit greater improvements in cardiovascular fitness and walking endurance among stroke patients.14 It can also help to improve balance and gait when targeted weight shifting, balance and coordination task interventions are used along with body weight support.15 A body weight support system allows for more targeted attention to the impaired areas while the patient remains secure and safe within a harness. This particular technology allows a therapist to work smarter and not harder while fine-tuning client care. It also allows a therapist to focus on the patient as a whole and supports use of multiple treatment modalities in a session. BWS reduces the need for a therapist to rely on multiple team members to treat a patient. This efficient approach also enables a therapist to spend more time on patient goals, which is ultimately what drives effective rehabilitation.
Functional Electrical Stimulation (FES)
Consistent and frequent intervention with FES can help in preventing further weakness that can result in additional issues and impairments, which can lead to further loss of function. FES can be performed for both the upper and the lower extremity through the use of FES orthotics / prosthetics, recumbent cycles, supine cycles, as well as with elliptical or stepping cycles. The use of FES has been shown to be equivalent to using an ankle foot orthosis.16,17 Additionally, task-specific training that includes parameters of body weight support training along with FES have been shown to be promising in improving gait coordination.18 Off cycle stimulation through an orthosis or handheld technology can also be utilized. FES on paretic lower limbs early after stroke improved the mobility and ability in ADLs.19 FES can also improve range of motion and has had a positive effect on muscle strength, cycling smoothness, maximal aerobic capacity, locomotion performance, postural control, spasticity, and motor coordination.20
Virtual Reality (VR)
To further support high frequency therapy, integration of therapies such as VR has proven important for improving patient engagement in therapies and patient experience.21 This technology is not limited to VR headsets but can also include video gaming systems. Using such technology can make therapy more relevant for the patient. Research has found video gaming and VR have had a significant impact on improving balance in individuals with stroke.22 Additionally, postural VR interventions as well as treadmill training with VR have shown positive impacts on timed up and go scores.23
Robot-Assisted Therapy
Robot-assisted therapy has been proposed as an excellent way to lower therapy costs by reducing the time spent with physiotherapists.24 Over the last several years, the study of lower extremity wearable exoskeletons has shown significant progress in supporting the function of walking for individuals with neuromuscular impairments. However, there continues to be a need for more quality studies for further clinical validation.25 Thus far, the studies involving lower extremity exoskeletons with post-stroke patients versus spinal cord injury and other neuromuscular conditions have shown the most reliable and promising results in terms of rehabilitation efficacy and favor robotic training over conventional gait therapy.26,27 Studies have found high evidence that the robot-assisted arm training improved ADLs, arm function, and arm strength in people after stroke.28 These studies also found evidence that robot-assisted arm training devices can be safe and acceptable to most participants. However, more research is required to determine the optimum use of these tools as existing studies vary in intensity, duration, and amount of training; type of treatment; participant characteristics; and measurements used.28
In-Clinic Rehabilitation Robots
Many robotic rehabilitation devices are still limited for use in the clinic. While these robots can offload therapists’ time burden, they generally cost tens of thousands of dollars and cannot yet assist with ADLs in the home.29-31 One design, an upper-limb exoskeleton, is being used to research the benefits of natural range of motion for the arm and shoulder.32 These robots may enable therapists to identify neuromuscular weakness and maladaptive coordination patterns and develop targeted interventions to address these aspects of upper-limb function. Another in-clinic upper extremity rehabilitation robot has demonstrated efficacy in rehabilitating the affected arm in individuals with chronic stroke over the course of 36 in-clinic sessions33 and in treating individuals with SCI.34
In-Home Powered Orthotic Solutions
Building upon the learnings of in-clinic rehabilitation robots, a new category of upper and lower extremity powered orthotics are being developed for in-home use. This allows for in-clinic gains to translate to home environments to ultimately improve functional performance and independence in ADLs. For the upper extremity, one such device is custom calibrated and fitted to patients to allow for independent movement to lift objects weighing up to 12 ounces.35,36 The level of caregiver support is recommended to be kept to a minimum in order to make the training challenging and maximize individual effort and contribution to movement by the patient.37 Integration of angle sensors at the shoulder and elbow capture duration of device usage and patient range of motion. Use of a passively powered approach allows the device to work with a patient’s natural strength without overriding it, which would lead to muscle atrophy. Supporting the upper extremity goes beyond the arm; inclusion of a supportive body frame with integrated lumbar sacral support (LSO) transfers the load of the device to the hips and torso to make the device feel weightless to the user and allow for extended use.35,36 A comfortable orthotic system design balances the load and torsion of assisted upper limb movement. Many patients have total body paralysis and require a stabilized trunk core to support dynamic upper limb movement.38-41
Gait Analysis Technologies
Pressure-sensitive electronic mats and walkways can be used to capture and compare objective measures of gait. They may be helpful not only in evaluating the gait characteristics of individuals who are recovering from stroke but in tracking progress and measuring the effectiveness of therapy. For example, walking speed data can be used to evaluate an individual’s functional status as well as predict recovery and help make decisions regarding therapeutic activities. Temporal, spatial, and pressure data can be captured to aid the therapist in making clinical assessments, with some systems capable of generating these data while an assistive device is used. The technologies are designed to be used with static standing, straight walking, turning, and other types of maneuvers, and are available in clinic-based and portable configurations.
Conclusion
The ultimate goal with stroke patients is to improve their quality of life, described by the Centers for Disease Control and Prevention in the US as “individual’s or group’s perceived physical and mental health over time.”42 There are multiple rehabilitation methods for treating stroke patients with reduced muscle function. Technology can be utilized on an ongoing basis to support these improvements. There are devices that are now approved for home use that can allow for increased opportunities for use with functional activities throughout the day, thus allowing the opportunity for improved quality of life. Technology can help engage the patient more fully in their rehabilitation for better patient compliance and adoption. This also allows for increased frequency of use, which can help potentially improve overall outcomes with stroke rehabilitation. RM
Kari Olk, PT, MSPT, is a physical therapist at HealthPartners in Minnesota. Her practice is located at Regions Hospital Rehabilitation – Neuroscience Center. HealthPartners is the largest consumer-governed, non-profit health care organization in the nation with a mission to improve health and well-being in partnership with members, patients, and the community.
Shawna Persaud, PhD, is director of clinical and product management at Abilitech Medical, which brings innovative solutions to market that allow people living with upper-limb neuromuscular conditions to function independently. The company’s first product is a powered orthotic device providing functional assistance and support to the elbow and shoulder to improve the lives of people in the US affected by neuromuscular weakness. For more information, contact [email protected].
References
- Woolley SM. Characteristics of gait in hemiplegia. Top Stroke Rehabil. 2001;7(4):1-18.
- Robbins SM, Houghton PE, Woodbury MG, Brown JL. The therapeutic effect of functional and transcutaneous electric stimulation on improving gait speed in stroke patients: a meta-analysis. Arch Phys Med Rehabil. 2006;87(6):853-9.
- Pizzi A, Carlucci G, Falsini C, Lunghi F, Verdesca S, Grippo A. Gait in hemiplegia: evaluation of clinical features with the Wisconsin Gait Scale. J Rehabil Med. 2007;39(2):170-4.
- Pereira S, Mehta S, McIntyre A, Lobo L, Teasell RW. Functional electrical stimulation for improving gait in persons with chronic stroke. Top Stroke Rehabil. 2012;19(6):491-8.
- Stewart JC, Cramer SC. Patient-reported measures provide unique insights into motor function after stroke. Stroke. 2013;44(4):1111-6.
- Post-Stroke Rehabilitation Fact Sheet | National Institute of Neurological Disorders and Stroke. 2020; Available from: https://www.ninds.nih.gov/Disorders/Patient-Caregiver-Education/Fact-Sheets/Post-Stroke-Rehabilitation-Fact-Sheet.
- Miller LE, Herbert WG. Health and economic benefits of physical activity for patients with spinal cord injury. Clinicoecon Outcomes Res. 2016;8:551-558.
- Peters HT, Page SJ, Persch A. Giving them a hand: wearing a myoelectric elbow-wrist-hand orthosis reduces upper extremity impairment in chronic stroke. Arch Phys Med Rehabil. 2017.
- McColl MA, Paterson M, Davies D, Doubt L, Law M. Validity and community utility of the Canadian Occupational Performance Measure. Can J Occup Ther. 2000;67(1):22-30.
- Langhorne P, Bernhardt J, Kwakkel G. Stroke rehabilitation. Lancet. 2011;377(9778):1693-702.
- Feys H, De Weerdt W, Verbeke G, et al. Early and repetitive stimulation of the arm can substantially improve the long-term outcome after stroke: a 5-year follow-up study of a randomized trial. Stroke. 2004;35(4):924-9.
- Patton J, Small SL, Rymer WZ, Functional restoration for the stroke survivor: informing the efforts of engineers. Top Stroke Rehabil. 2008;15(6):521-41.
- Pérez-de la Cruz S. Comparison between three therapeutic options for the treatment of balance and gait in stroke: a randomized controlled trial. Int J Environ Res Public Health. 2021;18(2).
14.Mackay-Lyons M, McDonald A, Matheson J, Eskes G, Klus M. Dual effects of body-weight supported treadmill training on cardiovascular fitness and walking ability early after stroke: a randomized controlled trial. Neurorehabil Neural Repair. 2013;27(7):644-53. - Bonan IV, Marquer A, Eskiizmirliler S, Yelnik AP, Vidal PP. Sensory reweighting in controls and stroke patients. Clin Neurophysiol. 2013;124(4):713-22.
- Turgut N, Möller L, Dengler K, et al. Adaptive cueing treatment of neglect in stroke patients leads to improvements in activities of daily living: a randomized controlled, crossover trial. Neurorehabil Neural Repair. 2018;32(11):988-998.
- Bethoux F, Rogers HL, Nolan KJ, et al. The effects of peroneal nerve functional electrical stimulation versus ankle-foot orthosis in patients with chronic stroke: a randomized controlled trial. Neurorehabil Neural Repair. 2014;28(7):688-97.
- Hollands KL, Pelton TA, Tyson SF, Hollands MA, van Vliet PM. Interventions for coordination of walking following stroke: systematic review. Gait Posture. 2012;35(3):349-59.
- You G, Liang H, Yan T. Functional electrical stimulation early after stroke improves lower limb motor function and ability in activities of daily living. NeuroRehabilitation. 2014;35(3):381-9.
- Barbosa D, Santos CP, Martins M. The application of cycling and cycling combined with feedback in the rehabilitation of stroke patients: a review. J Stroke Cerebrovasc Dis. 2015;24(2):253-73.
- Lee HC, Huang CL, Ho SH, Sung WH. The effect of a virtual reality game intervention on balance for patients with stroke: a randomized controlled trial. Games Health J. 2017;6(5):303-311.
- Ferreira V, Carvas N Jr, Artilheiro MC, Pompeu JE, Hassan SA, Kasawara KT. Interactive video gaming improves functional balance in poststroke individuals: meta-analysis of randomized controlled trials. Eval Health Prof. 2020;43(1):23-32.
- Iruthayarajah J, McIntyre A, Cotoi A, Macaluso S, Teasell R. The use of virtual reality for balance among individuals with chronic stroke: a systematic review and meta-analysis. Top Stroke Rehabil. 2017;24(1):68-79.
- Wagner TH, Lo AC, Peduzzi P, et al. An economic analysis of robot-assisted therapy for long-term upper-limb impairment after stroke. Stroke. 2011;42(9):2630-2.
- Rodríguez-Fernández A, Lobo-Prat J, Font-Llagunes JM. Systematic review on wearable lower-limb exoskeletons for gait training in neuromuscular impairments. J Neuroeng Rehabil. 2021;18(1):22.
- Bruni MF, Melegari C, De Cola MC, Bramanti A, Bramanti P, Calabro RS. What does best evidence tell us about robotic gait rehabilitation in stroke patients: A systematic review and meta-analysis. J Clin Neurosci. 2018;48:11-17.
- Mehrholz J, Thomas S, Werner C, Kugler J, Pohl M, Elsner B. Electromechanical-assisted training for walking after stroke. Cochrane Database Syst Rev. 2017;5(5):Cd006185.
- Mehrholz J, Pohl M, Platz T, Kugler J, Elsner B. Electromechanical and robot-assisted arm training for improving activities of daily living, arm function, and arm muscle strength after stroke. Cochrane Database Syst Rev. 2018;9:CD006876.
- Yanez-Sanchez A, Cuesta-Gomez A. Effectiveness of the Armeo(R) device in the rehabilitation of the upper limb of stroke’s patients. A review of the literature. Rev Neurol. 2020;70(3):93-102.
- Brewer BR, McDowell SK, Worthen-Chaudhari LC. Poststroke upper extremity rehabilitation: a review of robotic systems and clinical results. Top Stroke Rehabil. 2007;14(6):22-44.
- Marini F, Squeri V, Morasso P, Campus C, Konczak J, Masia L. Robot-aided developmental assessment of wrist proprioception in children. J Neuroeng Rehabil. 2017;14(1):3.
- Oliveira AC, Rose CG, Warburton K, et al. Exploring the capabilities of harmony for upper-limb stroke therapy. IEEE Int Conf Rehabil Robot. 2019:637-643.
- Colomer C, Baldovi A, Torrome A, Navarro MD, Moliner B, Noe E. Efficacy of Armeo(R) Spring during the chronic phase of stroke. Study in mild to moderate cases of hemiparesis. Neurologia. 2013;28(5):261-7.
- Rudhe C, Albisser U, Starkey ML, Curt A, Bolliger M. Reliability of movement workspace measurements in a passive arm orthosis used in spinal cord injury rehabilitation. J Neuroeng Rehabil. 2012;9(1):37.
- Abilitech Assist | Upper Arm Assist Device Powered Shoulder and Elbow. 2021; Available from: https://www.abilitechmedical.com/assist.
- Establishment Registration & Device Listing. 2021.
- Dietz V, Fouad K. Restoration of sensorimotor functions after spinal cord injury. Brain. 2014;137(Pt 3):654-67.
- Cabanas-Valdes R, Bagur-Calafat C, Girabent-Farres M, Caballero-Gomez FM, Hernandez-Valino M, Cuchi GU. The effect of additional core stability exercises on improving dynamic sitting balance and trunk control for subacute stroke patients: a randomized controlled trial. Clin Rehabil. 2016;30(10):1024-1033.
- Field-Fote EC, Ray SS. Seated reach distance and trunk excursion accurately reflect dynamic postural control in individuals with motor-incomplete spinal cord injury. Spinal Cord. 2010;48(10):745-9.
- Hirschfeld H. Motor control of every day motor tasks: guidance for neurological rehabilitation. Physiol Behav. 2007;92(1-2):161-6.
- Miyake Y, Kobayashi R, Kelepecz D, Nakajima M. Core exercises elevate trunk stability to facilitate skilled motor behavior of the upper extremities. J Bodyw Mov Ther. 2013;17(2):259-65.
- Health-Related Quality of Life (HRQOL) | CDC. 2018 2018-10-31T02:39:37Z/; Available from: https://www.cdc.gov/hrqol/index.htm.
This article was published in the 2021 March/April Rehab Management with the title, Repeat After Me.
Stair Training for Post-Stroke Strength
Stair walking training provides a task-oriented activity that helps improve leg strength and postural control for post-stroke individuals who are hemiparetic.1 Stair walking training can benefit both younger and older post-stroke populations.1 For example, among younger individuals it can effectively increase activity of the rectus femoris, while for elderly people it can increase muscle activity of the biceps femoris.2,3
The benefits of stair training are many-factored, but the height of the stairs is a variable that may have the greatest influence on the benefits of this activity. A recent study examined whether balance and muscle activity in the paretic lower limb would improve at stair heights of 10 centimeters and 15 centimeters.4 The study investigated whether the height differences would affect an individual’s level of function recovery.4
Height Matters
Study subjects self-selected their walking speed and walked over level ground as well as on a staircase and elevated walkway; one group testing stairs 10 centimeters in height and the other group testing stairs 15 centimeters in height. At the end of this 6-week intervention the muscle activities were the only data point that registered a significant difference between groups.
The researchers note that the 15-centimeter height group had higher rectus femoris, biceps femoris, and tibialis anterior activities than the 10-centimeter height group. According to the study leaders, the study data suggest stair walking training at a stair height of 15 centimeters more effectively increases the muscle activity of the paralyzed lower limb.
“Therefore,” the researchers conclude, “stair walking training at a stair height of 15 centimeters may be an effective intervention to improve the paralyzed leg strength of patients with stroke.”
Stairway to Confidence
Therapy clinics may turn to a variety of technologies to facilitate stair walking training, including corner exercise staircases and electronic stair steppers. Another option are dynamic stair trainers equipped with electronically elevating steps that are able to reach a height of up to 16.5 centimeters. One dynamic stair trainer on the market is designed with steps that can be adjusted in 1-centimeter increments so that patient can safely progress and build confidence. Whatever technology is used, stair walking training offers an effective activity to help stroke survivors reach a higher level of function. RM
References
- Stacoff A, Diezi C, Luder G, Stüssi E, Kramers-de Quervain IA. Ground reaction forces on stairs: effects of stair inclination and age. Gait Posture. 2005;21:24–38.
- Riener R, Rabuffetti M, Frigo C. Stair ascent and descent at different inclinations. Gait Posture. 2002;15:32–44.
- Dean CM, Richards CL, Malouin F. Task-related circuit training improves performance of locomotor tasks in chronic stroke: a randomized, controlled pilot trial. Arch Phys Med Rehabil. 2000;81:409–417.
- Kim KM, Shin JJ, Park HM, Lee DY. The effect of thigh muscle activity for stair walking and stepper strengthening exercise in university students. J Korea Academia-Industrial Cooper Soc. 2014;15:936–938.
- Min BC, Kim JH, Jeon KJ, Lee DH, Kim JS. EMG fatigue comparative study of stair ascending and descending. Korea Ind Sys Engineer. 2006;2:73–76.
- Yoon-Hee C, Kyoung K, Sang-Yong L, Yong-Jun C. Lower limb muscle activities and gain in balancing ability following two types of stair gait intervention in adult post-chronic stroke patients: A preliminary, randomized-controlled study. Turk J Phys Med Rehabil. 2020;66(1):17-23. Published 2020 Mar 3. doi: 10.5606/tftrd.2020.3335





