Determining the Impact of Riparian Wetlands on Nutrient Cycling, Storage and Export in Permeable Agricultural Catchments
Abstract
:1. Introduction
- Wetlands in these permeable catchments act as a sink for N and P under baseflow conditions, with high concentrations of dissolved organic N (DON) and P (DOP) accumulated in soil porewaters following plant assimilation of nitrate (NO3-N) and orthophosphate (PO4-P), plant dieback and microbial decomposition of dead organic matter.
- The wetland acts as a nutrient source under high flow conditions, with nutrient-rich porewater exported to the adjacent River Lambourn, predominantly in the form of DON and DOP.
- Wet-warm periods, with associated changes in soil redox status provide the optimum conditions for gaseous N flux from the wetland via denitrification, and for the desorption of P from the soil matrix and its subsequent flux in aqueous form to the adjacent River Lambourn.
- The wetland source/sink function varies both from baseflow to high flow conditions, and also from wet year to dry year, with greater rates of nutrient export to the adjacent Lambourn in wetter years, and greater retention of both N and P in the soil porewaters in drier years.
- the sub-surface structure, hydrological connectivity and function of the wetland using geophysical mapping and geochemical ratio techniques to identify the likely origins and residence times of waters within the wetland soil matrix as these varied in space and time. In combination these would allow identification of the potential location of biogeochemical cycling hotspots within the wetland.
- the nutrient cycling, retention and export behaviour in the wetland using a high temporal and spatial resolution sampling and analysis programme for all N species and P fractions flushed to and through the wetland and out to the adjacent aquifer and river, including the use of isotope ratios to discover direct evidence of the rates and locations of denitrification hotspots in the wetland. From this, we could then determine:
- the biogeochemical cycling hotspots within the wetland;
- the extent to which inorganic N and P ‘loss’ could be accounted for via denitrification, biotic uptake, and degradation of dead organic matter leading to the accumulation and subsequent flushing of dissolved organic nutrient fractions within soil porewaters; and
- the whole system nutrient attenuation behaviour and capacity of natural riparian wetlands in intensively farmed permeable catchments.
- Whether an alternative, lighter touch approach could effectively identify the wetland biogeochemical functional zones and likely wetland nutrient ‘removal’ efficiency by using geochemical, geophysical and natural abundance isotopic techniques, testing the outcomes from this approach against the fine-resolution but more time-consuming identification of these zones achieved in step 2.
2. Materials and Methods
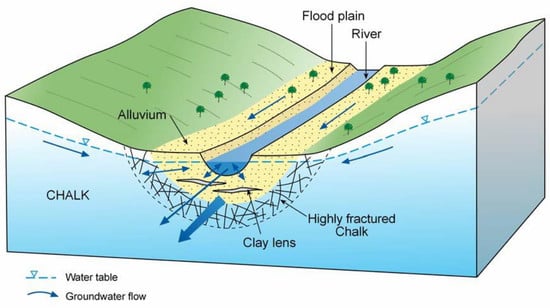
2.1. Site Description: The Boxford Wetland
2.2. Characterisation of Wetland Functional Zones Using Vegetation Mapping
2.3. Characterisation of Subsurface Stratigraphy Using Geophysical Techniques
2.4. Characterisation of the Biogeochemical Functional Zones Using Nutrient Speciation Chemistry, Major Ion Geochemistry and Isotope Geochemistry Analysis Approaches
2.4.1. Site Instrumentation
2.4.2. Samples Analysis to Determine N Species and P Fraction Concentrations in Porewaters
2.4.3. Sample Analysis to Determine Major Element Geochemistry
2.4.4. Sample Analysis to Determine Isotope Ratios in Soil Water, Alluvial Gravels and Wetland Vegetation
3. Results
3.1. Characterising Wetland Hydrological Function through Hydrological Monitoring and Geochemical Indices
- Groundwater nutrient chemistry is predominantly in the form of nitrate, with a mean concentration of 9 mg/L nitrate (NO3-N) and 1.5 mg/L DON in the Boxford borehole over the period 16 September 2003 to 16 January 2005. Similar chemistry is evident in the proto-stream, the River Lambourn and the piezometers at 2–3.5 m below the wetland. The data suggest that all of these sites share a similar source, and it is likely that they are all recently derived from groundwater sources.
- Mean DON concentrations in the River Lambourn, the piezometers and the proto-stream all show some enrichment over the 1.5 mg/L DON recorded at the Boxford borehole adjacent to the wetland, and this is likely to reflect mixing of groundwater with enriched soil porewaters draining from the wetland under baseflow conditions, and/or biogeochemical cycling within the River Lambourn [5,36,37,38].
- Samplers within the wetland soil matrix which are very close to the proto-stream channel also share this chemistry, notably those at C1 (20 cm), and at all three depths at D5. Sampler nest D5 is very close (<5 m) to the Boxford borehole, and lies outside the wetland proper, in the line of blackthorn scrub at the margins of the site. It may be that this indicates shared source water with that sampled from the borehole.
- Samplers further from the proto-stream channel, with a longer hydraulic residence time, show depletion of inorganic N under baseflow conditions, and this is likely to reflect both plant uptake and gaseous N loss through denitrification.
- These samples also show enrichment with DON concentrations up to a maximum of 4 mg/L.
- Samples collected at 60 cm depth along transect A show a chemistry similar to that of the adjacent River Lambourn, with a notable increase in nitrate concentrations from 20 to 60 cm depth at all stations along this transect. This may reflect riverine incursion from the Lambourn.
3.2. Identifying Biogeochemical Cycling Hotspots Using Isotope Geochemical Analysis
3.3. Developing a Conceptual Model of Wetland Biogeochemical Function under Baseflow versus Stormflow Conditions
4. Discussion
5. Conclusions
- Attenuation of pollutant delivery from land to stream appears to occur both within the hyporheic zone and the adjacent riparian wetland ecosystem.
- The primary pathway for modification of the nutrient speciation of inflowing waters is through plant uptake of inorganic nutrient species and microbiological breakdown of DOM to release DON and DOP compounds to soil porewaters.
- The reciprocity in inorganic and organic nutrient fraction concentrations across the wetland suggests no net storage of nutrients within the soil porewaters, nor substantial net export of nitrogen to atmospheric sources through denitrification.
- The primary mechanism for the export of nutrients accumulated in soil porewaters appears to be flushing of the macropores and micropores during storm events, with nutrient-rich waters exported primarily via the proto-stream channel, but also through lateral flow to the Lambourn and vertical exchange with groundwater through the alluvial gravels.
- The findings from the geochemical analyses of soil porewaters and source waters suggest that the wetland is groundwater fed, with flows likely to be delivered from the major dry valley feature which appears, topographically, to be aligned upslope with the line of the wetland proto-stream channel.
- The extent to which nutrient chemistry of inflowing waters is modified by wetland biogeochemical cycling depends on the residence time for the water within the wetland
- Well-drained areas of the wetland with a short hydraulic residence time exhibit soil porewater nutrient chemistry and major and trace element geochemistry comparable to chalk groundwater, suggesting little transformation of the nutrient load moving along these flow pathways.
- Surface and subsurface features identified through the site topographic and geophysical surveys, supplemented by soil coring, such as the existence and extent of the proto-stream channel, the depth of the alluvial gravels, and the presence of putty chalk lenses, are also critical in defining the hydrological function of wetland systems and the likely role any wetland may play in the transport and transformation of nutrient loads exported from land to stream.
- Geophysical methods, in association with limited site characterisation through geochemical, isotope and nutrient hydrochemical techniques may be useful in future studies to indicate the likely role played by wetlands in the nutrient hydrochemical function of permeable catchments, allowing them to be built into catchment scale models of biogeochemical function.
- This combination of techniques, building on recent advances in geophysical, geochemical and isotope characterisation provides a novel approach for improved understanding of biogeochemical function in permeable wetlands. Perhaps more importantly, no single technique on its own would have given an unambiguous representation of the hydrological and biogeochemical function of the wetland, and a toolkit comprising a range of complementary methods will provide a more complete and robust indication of wetland function at a process scale.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Peterjohn, W.T.; Correll, D.L. Nutrient dynamics in an agricultural watershed: Observations on the role of a riparian forest. Ecology 1984, 65, 1466–1475. [Google Scholar] [CrossRef]
- Seitzinger, S.P. Linkages between organic matter mineralization and denitrification in 8 riparian wetlands. Biogeochemistry 1994, 25, 19–39. [Google Scholar] [CrossRef]
- Hill, A.R. Nitrate removal in stream riparian zones. J. Environ. Qual. 1996, 25, 743–755. [Google Scholar] [CrossRef]
- Kuusemets, V.; Mander, U.; Lohmus, K.; Ivask, M. Nitrogen and phosphorus variation in shallow groundwater and assimilation in plants in complex riparian buffer zones. Water Sci. Technol. 2001, 44, 615–622. [Google Scholar] [CrossRef]
- Prior, H.; Johnes, P.J. Regulation of surface water quality in a Chalk catchment, UK: An assessment of the relative importance of instream and wetland processes. Sci. Total Environ. 2002, 282–283, 159–174. [Google Scholar] [CrossRef]
- Revsbech, N.P.; Jacobsen, J.P.; Nielsen, L.P. Nitrogen transformations in microenvironments of river beds and riparian zones. Ecol. Eng. 2005, 24, 447–455. [Google Scholar] [CrossRef]
- Burgin, A.J.; Groffman, P.M. Soil O2 controls denitrification rates and N2O yield in a riparian wetland. J. Geophys. Res. 2012, 117, G01010. [Google Scholar] [CrossRef] [Green Version]
- Arheimer, B.; Pers, B.C. Lessons learned? Effects of nutrient reductions from constructing wetlands in 1996–2006 across Sweden. Ecol. Eng. 2017, 103, 404–414. [Google Scholar] [CrossRef] [Green Version]
- Mander, U.; Tournebize, J.; Sauvage, S.; Sanchez-Perez, J.M. Wetlands and buffer zones in watershed management. Ecol. Eng. 2017, 103, 289–295. [Google Scholar] [CrossRef]
- Neilen, A.D.; Chen, C.R.; Parker, B.M.; Faggotter, S.J.; Burford, M.A. Differences in nitrate and phosphorus export between wooded and grassed riparian zones from farmland to receiving waterways under varying rainfall conditions. Sci. Total Environ. 2017, 598, 188–197. [Google Scholar] [CrossRef]
- Bernard-Jannin, L.; Sun, X.; Teissier, S.; Sauvage, S.; Sánchez-Pérez, J.M. Spatio-temporal analysis of factors controlling nitrate dynamics and potential denitrification hot spots and hot moments in groundwater of an alluvial floodplain. Ecol. Eng. 2017, 103, 272–284. [Google Scholar] [CrossRef] [Green Version]
- Peralta-Maraver, I.; Reiss, J.; Robertson, A.L. Interplay of hydrology, community ecology and pollutant attenuation in the hyporheic zone. Sci. Total Environ. 2018, 610–611, 267–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheliga, B.; Tetzlaff, D.; Nuetzmann, G.; Soulsby, C. Groundwater dynamics at the hillslope–riparian interface in a year with extreme winter rainfall. J. Hydrol. 2018, 564, 509–528. [Google Scholar] [CrossRef] [Green Version]
- Old, G.H.; Naden, P.S.; Rameshwaran, P.; Acreman, M.C.; Baker, S.; Edwards, F.K.; Sorensen, J.P.R.; Mountford, O.; Gooddy, D.C.; Stratford, C.J.; et al. Instream and riparian implications of weed cutting in a chalk river. Ecol. Eng. 2014, 71, 290–300. [Google Scholar] [CrossRef] [Green Version]
- Shabaga, J.A.; Hill, A.R. Groundwater-fed surface flow path hydrodynamics and nitrate removal in three riparian zones in southern Ontario, Canada. J. Hydrol. 2010, 388, 52–64. [Google Scholar] [CrossRef]
- Balestrini, R.; Sacchi, E.; Tidilia, D.; Deconte, C.A.; Buffagni, A. Factors affecting agricultural nitrogen removal in riparian strips: Examples from groundwater-dependent ecosystems of the Po Valley (Northern Italy). Agric. Ecosyst. Environ. 2016, 221, 132–144. [Google Scholar] [CrossRef]
- Pinay, G.; Decamps, H. The role of riparian woods in regulating nitrogen fluxes between the alluvial aquifer and surface water: A conceptual model. Regul. Rivers Res. Manag. 1988, 2, 507–516. [Google Scholar] [CrossRef]
- Hefting, M.M.; Bobbink, R.; de Caluwe, H. Nitrous oxide emission and denitrification in chronically nitrate-loaded riparian buffer zones. J. Environ. Qual. 2003, 32, 1194–1203. [Google Scholar] [CrossRef]
- Weyer, C.; Peiffer, S.; Lischeid, G. Stream water quality affected by interacting hydrological and biogeochemical processes in a riparian wetland. J. Hydrol. 2018, 563, 260–272. [Google Scholar] [CrossRef]
- Frei, S.; Peiffer, S. Exposure times rather than residence times control redox transformation efficiencies in riparian wetlands. J. Hydrol. 2016, 543, 182–196. [Google Scholar] [CrossRef]
- De Sosa, L.L.; Glanville, H.C.; Marshall, M.R.; Williams, P.; Abadie, M.; Clark, I.M.; Blaud, A.; Jones, D.L. Spatial zoning of microbial functions and plant-soil nitrogen dynamics across a riparian area in an extensively grazed livestock system. Soil Biol. Biochem. 2018, 120, 153–164. [Google Scholar] [CrossRef]
- Gutknecht, J.L.M.; Goodman, R.M.; Balser, T.C. Linking soil process and microbial ecology in freshwater wetland ecosystem. Plant Soil 2006, 289, 17–34. [Google Scholar] [CrossRef]
- Yates, C.A.; Johnes, P.J.; Spencer, R.G.M. Assessing the controls on dissolved organic matter export from two contrasting lowland catchments. Sci. Total Environ. 2016, 569–570, 1330–1340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lloyd, C.E.M.; Johnes, P.J.; Freer, J.E.; Carswell, A.; Jones, J.I.; Stirling, M.W.; Hodgkinson, R.A.; Richmond, C.; Collins, A.C. Determining the origins of nutrient flux to waters in catchments: Examining the nutrient speciation balance to inform the targeting of mitigation measures. Sci. Total Environ. 2019, 648, 1179–1200. [Google Scholar] [CrossRef] [PubMed]
- Yates, C.A.; Johnes, P.J.; Owen, A.T.; Brailsford, F.L.; Glanville, H.C.; Evans, C.D.; Marshall, M.R.; Jones, D.L.; Lloyd, C.E.M.; Jickells, T.; et al. Variation in dissolved organic matter (DOM) stoichiometry in freshwaters: Assessing the influence of land cover and soil C:N ratio on DOM composition. Limnol. Oceanogr. 2019, 9999, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Mander, U.; Kuusemets, V.; Ivask, M. Nutrient dynamics of riparian ecotones – a case study from the Porijogi River catchment, Estonia. Landsc. Urban Plan. 1995, 31, 333–348. [Google Scholar] [CrossRef]
- Kull, A.; Kull, A.; Jaagus, J.; Kuusemets, V.; Mander, U. The effects of fluctuating climatic conditions and weather events on nutrient dynamics in a narrow mosaic riparian peatland. Boreal Environ. Res. 2008, 13, 243–263. [Google Scholar]
- Newell, A.J.; Vane, C.H.; Sorensen, J.P.R.; Moss-Hayes, V.; Gooddy, D.C. Long-term Holocene groundwater fluctuations in a chalk catchment: Evidence from Rock-Eval pyrolysis of riparian peats. Hydrol. Process. 2016, 30, 4556–4567. [Google Scholar] [CrossRef] [Green Version]
- Brinson, M.M. A Hydrogeomorphic Classification for Wetlands; Wetlands Research Program Technical Report WRP-DE-4; US Army Corps of Engineers Waterways Experiment Station: Vicksburg, MS, USA, 1993. [Google Scholar]
- Allen, D.J.; Darling, W.G.; Gooddy, D.C.; Lapworth, D.J.; Newell, A.J.; Williams, A.T.; Allen, D.; Abesser, C. Interaction between groundwater, surface water and the hyporheic zone in a chalk stream. Hydrogeol. J. 2010, 18, 1125–1141. [Google Scholar] [CrossRef] [Green Version]
- Binley, A.; Hubbard, S.S.; Huisman, J.A.; Revil, A.; Robinson, D.A.; Singha, K.; Slater, L.D. The emergence of hydrogeophysics for improved understanding of subsurface processes over multiple scales. Water Resour. Res. 2015, 51, 3837–3866. [Google Scholar] [CrossRef] [Green Version]
- Uhlemann, S.S.; Sorensen, J.P.R.; House, A.R.; Wilkinson, P.B.; Roberts, C.; Gooddy, D.C.; Binley, A.; Chambers, J.E. Integrated time-lapse geoelectrical imaging of wetland hydrological processes. Water Resour. Res. 2016, 52, 1607–1625. [Google Scholar] [CrossRef] [Green Version]
- House, A.R.; Sorensen, J.P.R.; Gooddy, D.C.; Newell, A.J.; Marchant, B.; Mountford, J.O.; Scarlett, P.; Williams, P.J.; Old, G.H. Discrete wetland groundwater discharges revealed with a three-dimensional temperature model and botanical indicators (Boxford, UK). Hydrogeol. J. 2015, 23, 775–787. [Google Scholar] [CrossRef] [Green Version]
- Wheater, H.S.; Peach, D. Developing interdisciplinary science for integrated catchment management: The UK Lowland Catchment Research (LOCAR) programme. Int. J. Water Resour. Dev. 2004, 20, 369–385. [Google Scholar] [CrossRef] [Green Version]
- Wheater, H.; Peach, D.; Binley, A. Characterising groundwater-dominated lowland catchments: The UK Lowland Catchment Research Programme (LOCAR). Hydrol. Earth Syst. Sci. 2007, 11, 108–124. [Google Scholar] [CrossRef]
- Evans, D.J.; Johnes, P.J.; Lawrence, D.S. Sediment Transport in Lowland Streams—Storm Integrated Monitoring; IAHS Publication: Wallingford, UK, 2003; Volume 283, pp. 103–110. [Google Scholar]
- Evans, D.J.; Johnes, P.J. Physico-chemical controls on phosphorus cycling in two lowland streams: I—Water column. Sci. Total Environ. 2004, 329, 145–163. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.J.; Johnes, P.J.; Lawrence, D. Physico-chemical controls on phosphorus cycling in two lowland streams: II—Bed sediment. Sci. Total Environ. 2004, 329, 165–182. [Google Scholar] [CrossRef] [PubMed]
- Lapworth, D.J.; Gooddy, D.C.; Allen, D.; Old, G.H. Understanding groundwater, surface water, and hyporheic zone biogeochemical processes in a Chalk catchment using fluorescence properties of dissolved and colloidal organic matter. J. Geophys. Res. 2009, 114, G00F02. [Google Scholar] [CrossRef] [Green Version]
- Grapes, T.R.; Bradley, C.; Petts, G.E. Dynamics of river-aquifer interaction along a Chalk stream: The River Lambourn, UK. Hydrol. Process. 2005, 19, 2035–2053. [Google Scholar] [CrossRef]
- Griffiths, J.; Binley, A.; Crook, N.; Nutter, J.; Young, A.; Fletcher, S. Streamflow generation in the Pang and Lambourn catchments, Berkshire, UK. J. Hydrol. 2006, 330, 71–83. [Google Scholar] [CrossRef]
- Newell, A.J.; Sorensen, J.P.R.; Chambers, J.E.; Wilkinson, P.B.; Uhleman, S.; Roberts, C.; Gooddy, D.C.; Vane, C.H.; Binley, A. Fluvial response to Late Pleistocene and Holocene environmental change in a Thames chalkland headwater: The Lambourn of southern England. Proc. Geol. Assoc. 2015, 126, 683–697. [Google Scholar] [CrossRef] [Green Version]
- Johnes, P.J.; Foy, R.; Butterfield, D.; Haygarth, P.M. Land use scenarios for England and Wales: Evaluation of management options to support ‘good ecological status’ in surface freshwaters. Soil Use Manag. 2007, 23, 176–194. [Google Scholar] [CrossRef]
- Pretty, J.L.; Hildrew, A.G.; Trimmer, M. Nutrient dynamics in relation to surface–subsurface hydrological exchange in a groundwater fed Chalk stream. J. Hydrol. 2006, 330, 84–100. [Google Scholar] [CrossRef]
- Lapworth, D.J.; Gooddy, D.C.; Jarvie, H.P. Understanding phosphorus mobility and bioavailability in the hyporheic zone of a chalk stream. Water Air Soil Pollut. 2011, 218, 213–226. [Google Scholar] [CrossRef]
- Aldiss, D.T.; Royse, K.T. The geology of the Pang-Lambourn catchment, Berkshire: British Geological Survey Report CR/20/289N; British Geological Survey: Keyworth, Nottingham, UK, 2002; p. 38. [Google Scholar]
- Rodwell, J.S. National Vegetation Classification: Users’ Handbook; Joint Nature Conservation Committee: Peterborough, UK, 2006.
- Kettridge, N.; Comas, X.; Baird, A.; Slater, L.; Strack, M.; Thompson, D.; Jol, H.; Binley, A. Ecohydrologically important subsurface structures in peatlands revealed by ground-penetrating radar and complex conductivity surveys. J. Geophys. Res. 2008, 113, G04030. [Google Scholar] [CrossRef] [Green Version]
- Robinson, D.A.; Binley, A.; Crook, N.; Day-Lewis, F.; Ferré, P.T.; Grauch, V.J.S.; Knight, R.; Knoll, M.; Lakshmi, V.; Miller, R.; et al. Advancing process-based watershed hydrological research using near-surface geophysics: A vision for, and review of, electrical and magnetic geophysical methods. Hydrol. Process. 2008, 22, 3604–3635. [Google Scholar] [CrossRef]
- McLachlan, P.J.; Chambers, J.E.; Uhlemann, S.S.; Binley, A. Geophysical characterisation of the groundwater—Surface water interface. Adv. Water Resour. 2017, 109, 302–319. [Google Scholar] [CrossRef] [Green Version]
- Musgrave, H.; Binley, A. Revealing the temporal dynamics of subsurface temperature in a wetland using time-lapse geophysics. J. Hydrol. 2011, 396, 258–266. [Google Scholar] [CrossRef]
- Binley, A.; Kemna, A. DC Resistivity and Induced Polarization Methods. In Hydrogeophysics; Rubin, Y., Hubbard, S.S., Eds.; Springer: New York, NY, USA, 2005; pp. 129–156. [Google Scholar]
- Jarvis, I.; Woodruff, P. The phosphatic chalks and hardgrounds of Boxford and Winterbourne, Berkshire—Two tectonically controlled facies in the late Coniacian to early Campanian (Cretaceous) of southern England. Geol. Mag. 1981, 118, 175–187. [Google Scholar] [CrossRef]
- Johnes, P.J.; Heathwaite, A.L. A procedure for the simultaneous determination of total nitrogen and total phosphorus in freshwater samples using persulphate microwave digestion. Water Res. 1992, 26, 1281–1287. [Google Scholar] [CrossRef]
- Cey, E.E.; Rudolph, D.L.; Aravena, R.; Parkin, G. Role of the riparian zone in controlling the distribution and fate of agricultural nitrogen near a small stream in southern Ontario. J. Contam. Hydrol. 1999, 37, 45–67. [Google Scholar] [CrossRef]
- Mengis, M.; Schiff, S.L.; Haris, M.; English, M.C.; Aravena, R.; Elgood, R.J.; MacLean, A. Multiple geochemical and isotopic approaches for assessing ground water NO3− elimination in a riparian zone. Groundwater 1999, 37, 448–457. [Google Scholar] [CrossRef]
- Devito, K.J.; Fitzgerald, D.; Hill, A.R.; Aravena, R. Nitrate dynamics in relation to lithology and hydrologic flow path in a river riparian zone. J. Environ. Qual. 2000, 29, 1075–1084. [Google Scholar] [CrossRef]
- Chang, C.C.Y.; Langston, J.; Riggs, M.; Campbell, D.H.; Silva, S.R.; Kendall, C. A method for nitrate collection for δ15N and δ18O analysis from waters with low nitrate concentrations. Can. J. Fish. Aquat. Sci. 1999, 56, 1856–1864. [Google Scholar] [CrossRef]
- Heaton, T.H.E.; Wynn, P.; Tye, A. Low 15N/14N ratios for nitrate in snow in the High Arctic (79o N). Atmos. Environ. 2004, 38, 5611–5621. [Google Scholar] [CrossRef]
- Mullinger, N.J.; Pates, J.M.; Binley, A.; Crook, N.P. Controls on the spatial and temporal variability of 222Rn-in riparian groundwater in a lowland Chalk catchment. J. Hydrol. 2009, 376, 58–69. [Google Scholar] [CrossRef] [Green Version]
- Cook, P.G.; Wood, C.; White, T.; Simmons, C.T.; Fass, T.; Brunner, P. Groundwater inflow to a shallow, poorly mixed wetland estimated from a mass balance of radon. J. Hydrol. 2008, 354, 213–226. [Google Scholar] [CrossRef]
- Massmann, G.; Sultenfuss, J.; Dunnbier, U.; Knappe, A.; Taute, T.; Pekdeger, A. Investigation of groundwater residence times during bank filtration in Berlin: A multi-tracer approach. Hydrol. Process. 2008, 22, 788–801. [Google Scholar] [CrossRef]
- Christensen, J.N.; Dafflon, B.; Shiel, A.E.; Tokunaga, T.K.; Wan, J.; Faybishenko, B.; Dong, W.; Williams, K.H.; Hobson, C.; Brown, S.T.; et al. Using strontium isotopes to evaluate the spatial variation of groundwater recharge. Sci. Total Environ. 2018, 637–638, 672–685. [Google Scholar] [CrossRef] [Green Version]
- Gooddy, D.C.; Darling, W.G.; Abesser, C.; Lapworth, D.J. Using chlorofluorocarbons (CFCs) and sulphur hexafluoride (SF6) to characterise groundwater movement and residence time in a lowland chalk catchment. J. Hydrol. 2006, 330, 44–52. [Google Scholar] [CrossRef] [Green Version]
- Chambers, D.; Gooddy, D.C.; Binley, A. Use and application of CFC-11, CFC-12, CFC-113 and SF6 as environmental tracers of groundwater residence time: A review. Geosci. Front. 2019, 10, 1643–1652. [Google Scholar] [CrossRef]
- Sigman, D.M.; Casciotti, K.L.; Andreani, M.; Barford, C.; Galanter, M.; Böhlke, J.K. A bacterial method for the nitrogen isotopic analysis of nitrate in seawater and freshwater. Anal. Chem. 2001, 73, 4145–4153. [Google Scholar] [CrossRef] [PubMed]
- Feast, N.A.; Hiscock, K.M.; Dennis, P.F.; Andrews, J.N. Nitrogen isotope hydrochemistry and denitrification within the chalk aquifer system of north Norfolk, UK. J. Hydrol. 1998, 211, 233–252. [Google Scholar] [CrossRef]
- Kendall, C.; Elliott, E.M.; Wankel, S.D. Tracing anthropogenic inputs of nitrogen to ecosystems. In Stable Isotopes in Ecology and Environmental Science; Michener, R., Lajtha, K., Eds.; Blackwell Publishing: Oxford, UK, 2007; pp. 375–449. [Google Scholar]
- Luz, B.; Barkan, E. The isotopic composition of atmospheric oxygen. Glob. Biogeochem. Cycles 2011, 25, GB4006. [Google Scholar] [CrossRef]
- Chen, D.J.Z.; Macquarrie, K.T.B. Correlation of δ15N and δ18O in NO3− during denitrification in groundwater. J. Environ. Eng. Sci. 2005, 4, 221–226. [Google Scholar] [CrossRef]
- Heaton, T.H.E.; Stuart, M.E.; Sapiano, M.; Micallef Sultana, M. An isotope study of the sources of nitrate in Malta’s groundwater. J. Hydrol. 2012, 414–415, 244–254. [Google Scholar] [CrossRef]
- Green, C.T.; Böhlke, J.K.; Bekins, B.A.; Phillips, S.P. Mixing effects on apparent reaction rates and isotope fractionation during denitrification in a heterogeneous aquifer. Water Resour. Res. 2010, 46, W08525. [Google Scholar] [CrossRef]
- Evans, R.D. Physiological mechanisms influencing plant nitrogen isotope composition. Trends Plant Sci. 2001, 6, 122–126. [Google Scholar] [CrossRef]














| Sampling Programme | Dates |
|---|---|
| Weekly Water Sampling: | |
| Water level at dipwells | 16 September 2003–16 January 2005 |
| Soil porewaters in wetland alluvial soils, 23 nests, 20, 40, 60 cm | 16 September 2003–16 January 2005 |
| Water levels in wetland alluvial soils, 23 dipwells, | 7 January 2004–16 January 2005 |
| Proto-stream surface water, 3 sites, | 7 January 2004–16 July 2004 |
| Boxford borehole, 30 m depth | 1 December 2004–16 January 2005 |
| Piezometers in gravels, 5 sites, 2 m, 3 m depth | 1 July 2004–16 January 2005 |
| Puncture tensiometers in wetland alluvial soils | 1 September 2004–16 January 2005 |
| Plant Sampling: | |
| Plant species list | April 2004, June 2004 |
| Biomass sampling, single and mixed species stands, monthly | Monthly |
| Structural measurements, including canopy height, stem density per unit area, average stem diameter recorded monthly | Monthly |
| Plant health monitored monthly using fluorimeter | Monthly |
| Wetland Soil Porewater and Source Water Geochemistry: | |
| Soil porewaters, piezometers, river and groundwater sampled twice and analysed to determine geochemical signature. | July 2004, December 2004 |
| Isotopic Signatures of Wetland Biogeochemical Function: | |
| 15N/14N and 18O/16O determined on water samples collected from porous cup samplers along transects, piezometers, borehole, river | October 2004 |
| 15N/14N determined on vegetation samples collected along transects | October 2004 |
| Sites Sampled | δ15N (‰ vs. AIR) | δ18O (‰ vs. SMOW) | NO3-N mg/L |
|---|---|---|---|
| Boreholes | |||
| Westbrook array | |||
| PL26 D-1, SO4-01126 | +3.9 | +2 | 5.84 |
| PL26 D-2, SO4-01127 | +4.0 | +6 | 6.29 |
| PL26 N-4, SO4-01131 | +4.3 | +1 | 6.87 |
| PL26 H-2, SO4-01134 | +3.9 | +5 | 6.09 |
| PL26 E-2, SO4-01138 | +4.1 | +3 | 6.05 |
| Boxford borehole (sampled 02-October-2004) | +4.0 | +3 | |
| Boxford wetland (sampled 27-October-2004) | |||
| B2 ‘short’ piezometer | +4.5 | +2 | 5.76 |
| C1 piezometer | +4 | 4.70 | |
| C1 proto-stream lower | +5.0 | +3 | 5.58 |
| C5 piezometer | +4.6 | +3 | 6.67 |
| D1 proto-stream main | +4.8 | +1 | 5.60 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johnes, P.J.; Gooddy, D.C.; Heaton, T.H.E.; Binley, A.; Kennedy, M.P.; Shand, P.; Prior, H. Determining the Impact of Riparian Wetlands on Nutrient Cycling, Storage and Export in Permeable Agricultural Catchments. Water 2020, 12, 167. https://doi.org/10.3390/w12010167
Johnes PJ, Gooddy DC, Heaton THE, Binley A, Kennedy MP, Shand P, Prior H. Determining the Impact of Riparian Wetlands on Nutrient Cycling, Storage and Export in Permeable Agricultural Catchments. Water. 2020; 12(1):167. https://doi.org/10.3390/w12010167
Chicago/Turabian StyleJohnes, Penny J., Daren C. Gooddy, Timothy H. E. Heaton, Andrew Binley, Michael P. Kennedy, Paul Shand, and Hannah Prior. 2020. "Determining the Impact of Riparian Wetlands on Nutrient Cycling, Storage and Export in Permeable Agricultural Catchments" Water 12, no. 1: 167. https://doi.org/10.3390/w12010167