Accessing the Life in Smoke: A New Application of Unmanned Aircraft Systems (UAS) to Sample Wildland Fire Bioaerosol Emissions and Their Environment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Prescribed Burns
2.3. Aerobiological Sampling
2.4. Particulate Matter and Meteorology Sampling
2.5. Microbial Cultures and Identification
2.6. Statistical Analyses
3. Results
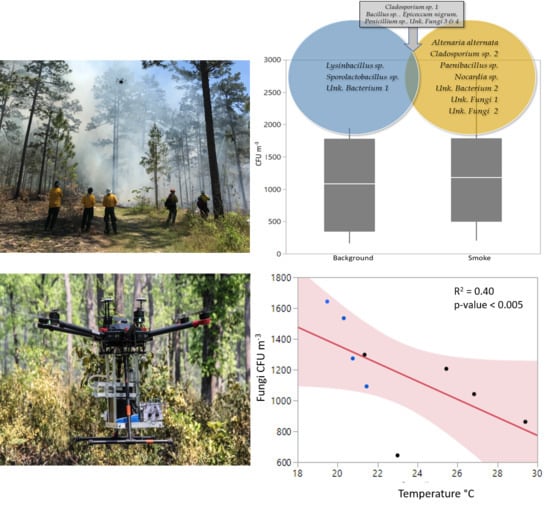
3.1. Microbial Presence in Smoke and Background Air
3.2. Relationship to Environmental Factors and Particulate Matter
4. Discussion
4.1. Sampling Bioaerosols from Aircraft Platforms
4.2. Species Composition and Abundance
4.3. Relationships of Bioaerosols and Environmental Factors, Including Particulate Matter
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Andreae, M.O.; Merlet, P. Emission of trace gases and aerosols from biomass burning. Glob. Biogeochem. Cycles 2001, 15, 955–966. [Google Scholar] [CrossRef]
- Jaenicke, R. Abundance of Cellular Material and Proteins in the Atmosphere. Science 2005, 308, 73. [Google Scholar] [CrossRef] [PubMed]
- Charland, A.M.; Clements, C.B. Kinematic structure of a wildland fire plume observed by Doppler lidar. J. Geophys. Res. Atmos. 2013, 118, 3200–3212. [Google Scholar] [CrossRef]
- O’Brien, J.J.; Loudermilk, E.L.; Hornsby, B.; Hudak, A.T.; Bright, B.C.; Dickinson, M.B.; Hiers, J.K.; Teske, C.; Ottmar, R.D. High-resolution infrared thermography for capturing wildland fire behaviour: RxCADRE 2012. Int. J. Wildland Fire 2016, 25, 62–75. [Google Scholar] [CrossRef]
- O’Brien, J.J.; Hiers, J.K.; Varner, J.M.; Hoffman, C.M.; Dickinson, M.B.; Michaletz, S.T.; Loudermilk, E.L.; Butler, B.W. Advances in Mechanistic Approaches to Quantifying Biophysical Fire Effects. Current. Forest. Report. 2018, 4, 161–177. [Google Scholar] [CrossRef]
- Kobziar, L.; Moghaddas, J.; Stephens, S.L. Tree mortality patterns following prescribed fires in a mixed conifer forest. Can. J. For. Res. 2006, 36, 3222–3238. [Google Scholar] [CrossRef]
- Pingree, M.R.A.; Kobziar, L.N. The myth of the biological threshold: A review of biological responses to soil heating associated with wildland fire. For. Ecol. Manag. 2019, 432, 1022–1029. [Google Scholar] [CrossRef]
- Glassman, S.I.; Levine, C.R.; DiRocco, A.M.; Battles, J.J.; Bruns, T.D. Ectomycorrhizal fungal spore bank recovery after a severe forest fire: Some like it hot. ISME J. 2016, 10, 1228–1239. [Google Scholar] [CrossRef]
- Polymenakou, P.N.; Polymenakou, P.N. Atmosphere: A Source of Pathogenic or Beneficial Microbes? Atmosphere 2012, 3, 87–102. [Google Scholar] [CrossRef]
- Fröhlich-Nowoisky, J.; Burrows, S.M.; Xie, Z.; Engling, G.; Solomon, P.A.; Fraser, M.P.; Mayol-Bracero, O.L.; Artaxo, P.; Begerow, D.; Conrad, R.; et al. Biogeography in the air: Fungal diversity over land and oceans. Biogeosciences 2012, 9, 1125–1136. [Google Scholar] [CrossRef]
- Lerner, P.I. Nocardiosis. Clin. Infect. Dis. 1996, 22, 891–903. [Google Scholar] [CrossRef]
- Brown, G.D.; Denning, D.W.; Gow, N.A.R.; Levitz, S.M.; Netea, M.G.; White, T.C. Hidden Killers: Human Fungal Infections. Sci. Transl. Med. 2012, 4, 165rv13. [Google Scholar] [CrossRef]
- Sharma, D.; Dutta, B.K.; Singh, A.B.; Shome, B.R. Aerobiological, biochemical and immunological studies on some of the dominant Aspergillus species of South Assam (India). Aerobiologia 2007, 23, 201. [Google Scholar] [CrossRef]
- Schmale, D.G.; Ross, S.D. Highways in the sky: Scales of atmospheric transport of plant pathogens. Annu. Rev. Phytopathol. 2015, 53, 591–611. [Google Scholar] [CrossRef]
- Hoose, C.; Kristjánsson, J.E.; Burrows, S.M. How important is biological ice nucleation in clouds on a global scale? Environ. Res. Lett. 2010, 5, 024009. [Google Scholar] [CrossRef]
- Hara, K.; Zhang, D. Bacterial abundance and viability in long-range transported dust. Atmos. Environ. 2012, 47, 20–25. [Google Scholar] [CrossRef]
- Smith, D.J.; Jaffe, D.A.; Birmele, M.N.; Griffin, D.W.; Schuerger, A.C.; Hee, J.; Roberts, M.S. Free Tropospheric Transport of Microorganisms from Asia to North America. Microb. Ecol. 2012, 64, 973–985. [Google Scholar] [CrossRef]
- Franzetti, A.; Gandolfi, I.; Gaspari, E.; Ambrosini, R.; Bestetti, G. Seasonal variability of bacteria in fine and coarse urban air particulate matter. Appl. Microbiol. Biotechnol. 2011, 90, 745–753. [Google Scholar] [CrossRef]
- Haas, D.; Galler, H.; Luxner, J.; Zarfel, G.; Buzina, W.; Friedl, H.; Marth, E.; Habib, J.; Reinthaler, F.F. The concentrations of culturable microorganisms in relation to particulate matter in urban air. Atmos. Environ. 2013, 65, 215–222. [Google Scholar] [CrossRef]
- Mims, S.A.; Mims, F.M. Fungal spores are transported long distances in smoke from biomass fires. Atmos. Environ. 2004, 38, 651–655. [Google Scholar] [CrossRef]
- Camacho, I.; Góis, A.; Camacho, R.; Nóbrega, V. Fernandez The impact of urban and forest fires on the airborne fungal spore aerobiology. Aerobiologia 2018, 34, 585–592. [Google Scholar] [CrossRef]
- Kobziar, L.N.; Pingree, M.R.A.; Larson, H.; Dreaden, T.J.; Green, S.; Smith, J.A. Pyroaerobiology: The aerosolization and transport of viable microbial life by wildland fire. Ecosphere 2018, 9, e02507. [Google Scholar] [CrossRef]
- Rajput, P.; Anjum, M.H.; Gupta, T. One year record of bioaerosols and particles concentration in Indo-Gangetic Plain: Implications of biomass burning emissions to high-level of endotoxin exposure. Environ. Pollut. 2017, 224, 98–106. [Google Scholar] [CrossRef]
- Yang, Y.; Chan, C.; Tao, J.; Lin, M.; Engling, G.; Zhang, Z.; Zhang, T.; Su, L. Observation of elevated fungal tracers due to biomass burning in the Sichuan Basin at Chengdu City, China. Sci. Total Environ. 2012, 431, 68–77. [Google Scholar] [CrossRef]
- Hirst, J.M. An Automatic Volumetric Spore Trap. Ann. Appl. Biol. 1952, 39, 257–265. [Google Scholar] [CrossRef]
- Lin, B.; Bozorgmagham, A.; Ross, S.D.; Schmale, D.G., III. Small fluctuations in the recovery of fusaria across consecutive sampling intervals with unmanned aircraft 100 m above ground level. Aerobiologia 2013, 29, 45–54. [Google Scholar] [CrossRef]
- West, J.; Kimber, R. Innovations in air sampling to detect plant pathogens. Ann. Appl. Biol. 2015, 166, 4–17. [Google Scholar] [CrossRef]
- Fernández-Rodríguez, S.; Sadyś, M.; Smith, M.; Tormo-Molina, R.; Skjøth, C.A.; Maya-Manzano, J.M.; Silva-Palacios, I.; Gonzalo-Garijo, Á. Potential sources of airborne Alternaria spp. spores in South-west Spain. Sci. Total Environ. 2015, 533, 165–176. [Google Scholar] [CrossRef]
- Haig, C.W.; Mackay, W.G.; Walker, J.T.; Williams, C. Bioaerosol sampling: Sampling mechanisms, bioefficiency and field studies. J. Hosp. Infect. 2016, 93, 242–255. [Google Scholar] [CrossRef]
- Villa, T.F.; Gonzalez, F.; Miljievic, B.; Ristovski, Z.D.; Morawska, L. An Overview of Small Unmanned Aerial Vehicles for Air Quality Measurements: Present Applications and Future Prospectives. Sensors 2016, 16, 1072. [Google Scholar] [CrossRef]
- Nelson, K.; Boehmler, J.; Khlystov, A.; Moosmüller, H.; Samburova, V.; Bhattarai, C.; Wilcox, E.; Watts, A. A Multipollutant Smoke Emissions Sensing and Sampling Instrument Package for Unmanned Aircraft Systems: Development and Testing. Fire 2019, 2, 32. [Google Scholar] [CrossRef] [Green Version]
- Haddrell, A.E.; Thomas, R.J. Aerobiology: Experimental Considerations, Observations, and Future Tools. Appl. Environ. Microbiol. 2017, 83, e00809–e00817. [Google Scholar] [CrossRef] [Green Version]
- Macher, J.M. Positive-hole correction of multiple-jet impactors for collecting viable microorganisms. Am. Ind. Hyg. Assoc. J. 1989, 50, 561–568. [Google Scholar] [CrossRef]
- Grinshpun, S.A.; Willeke, K.; Ulevicius, V.; Juozaitis, A.; Terzieva, S.; Donnelly, J.; Stelma, G.N.; Brenner, K.P. Effect of Impaction, Bounce and Reaerosolization on the Collection Efficiency of Impingers. Aerosol Sci. Technol. 1997, 26, 326–342. [Google Scholar] [CrossRef]
- Dybwad, M.; Skogan, G.; Blatny, J.M. Comparative Testing and Evaluation of Nine Different Air Samplers: End-to-End Sampling Efficiencies as Specific Performance Measurements for Bioaerosol Applications. Aerosol Sci. Technol. 2014, 48, 282–295. [Google Scholar] [CrossRef]
- Buttner, M.P.; Stetzenbach, L.D. Evaluation of Four Aerobiological Sampling Methods for the Retrieval of Aerosolized Pseudomonas syringae. Appl. Environ. Microbiol. 1991, 57, 1268–1270. [Google Scholar]
- Jimenez-Sanchez, C.; Hanlon, R.; Aho, K.A.; Powers, C.; Morris, C.E.; Schmale, D.G. Diversity and Ice Nucleation Activity of Microorganisms Collected With a Small Unmanned Aircraft System (sUAS) in France and the United States. Front. Microbiol. 2018, 9, 1667. [Google Scholar] [CrossRef] [Green Version]
- Powers, C.W.; Hanlon, R.; Grothe, H.; Prussin, A.J.; Marr, L.C.; Schmale, D.G. Coordinated Sampling of Microorganisms Over Freshwater and Saltwater Environments Using an Unmanned Surface Vehicle (USV) and a Small Unmanned Aircraft System (sUAS). Front. Microbiol. 2018, 9, 1668. [Google Scholar] [CrossRef] [Green Version]
- Vitorino, L.; Bessa, L. Microbial Diversity: The Gap between the Estimated and the Known. Diversity 2018, 10, 46. [Google Scholar] [CrossRef] [Green Version]
- Bush, R.K.; Prochnau, J.J. Alternaria-induced asthma. J. Allergy Clin. Immunol. 2004, 113, 227–234. [Google Scholar] [CrossRef]
- Mahadevakumar, S.; Jayaramaiah, K.M.; Janardhana, G.R. First Report of Leaf Spot Disease Caused by Epicoccum nigrum on Lablab purpureus in India. Plant Dis. 2014, 98, 284. [Google Scholar] [CrossRef] [PubMed]
- de Fávaro, L.C.L.; de Sebastianes, F.L.S.; Araújo, W.L. Epicoccum nigrum P16, a Sugarcane Endophyte, Produces Antifungal Compounds and Induces Root Growth. PLoS ONE 2012, 7, e36826. [Google Scholar] [CrossRef] [PubMed]
- Kloepper, J.W.; Leong, J.; Teintze, M.; Schroth, M.N. Pseudomonas siderophores: A mechanism explaining disease-suppressive soils. Curr. Microbiol. 1980, 4, 317–320. [Google Scholar] [CrossRef]
- Conen, F.; Morris, C.E.; Leifeld, J.; Yakutin, M.V.; Alewell, C. Biological residues define the ice nucleation properties of soil dust. Atmos. Chem. Phys. 2011, 11, 9643–9648. [Google Scholar] [CrossRef] [Green Version]
- Fröhlich-Nowoisky, J.; Kampf, C.J.; Weber, B.; Huffman, J.A.; Pöhlker, C.; Andreae, M.O.; Lang-Yona, N.; Burrows, S.M.; Gunthe, S.S.; Elbert, W.; et al. Bioaerosols in the Earth system: Climate, health, and ecosystem interactions. Atmos. Res. 2016, 182, 346–376. [Google Scholar] [CrossRef] [Green Version]
- Petters, M.D.; Parsons, M.T.; Prenni, A.J.; DeMott, P.J.; Kreidenweis, S.M.; Carrico, C.M.; Sullivan, A.P.; McMeeking, G.R.; Levin, E.; Wold, C.E.; et al. Ice nuclei emissions from biomass burning. J. Geophys. Res. Atmospheres 2009, 114, D07209. [Google Scholar] [CrossRef] [Green Version]
- Fan, X.-Y.; Gao, J.-F.; Pan, K.-L.; Li, D.-C.; Dai, H.-H.; Li, X. More obvious air pollution impacts on variations in bacteria than fungi and their co-occurrences with ammonia-oxidizing microorganisms in PM2.5. Environ. Pollut. 2019, 251, 668–680. [Google Scholar] [CrossRef]
- Almeida, E.; Caeiro, E.; Todo-Bom, A.; Ferro, R.; Dionísio, A.; Duarte, A.; Gazarini, L. The influence of meteorological parameters on Alternaria and Cladosporium fungal spore concentrations in Beja (Southern Portugal): Preliminary results. Aerobiologia 2018, 34, 219–226. [Google Scholar] [CrossRef]
- Healy, D.A.; Huffman, J.A.; O’Connor, D.J.; Pöhlker, C.; Pöschl, U.; Sodeau, J.R. Ambient measurements of biological aerosol particles near Killarney, Ireland: A comparison between real-time fluorescence and microscopy techniques. Atmos. Chem. Phys. 2014, 14, 8055–8069. [Google Scholar] [CrossRef] [Green Version]
- Kellogg, C.A.; Griffin, D.W. Aerobiology and the global transport of desert dust. Trends Ecol. Evolut. 2006, 21, 638–644. [Google Scholar] [CrossRef]


| Sample Type | Date | Time of Sampling (EDT) | Flow Rate (L min−1) | Duration (min) | Volume (L) | Height (m AGL) | Air Temperature (°C) | RH % | Windspeed (mph) |
|---|---|---|---|---|---|---|---|---|---|
| UAS, Smoke 1 | 19 April 2018 | 13:37 | 11.5 | <1 | 4.8 | 25 | 26.8 | 47.9 | NE 5-15 gusts |
| UAS, Smoke 2 | 19 April 2018 | 15:07 | 11.5 | <1 | 5.8 | 25 | 29.4 | 40.5 | NE 5- 15 gusts |
| UAS, Smoke 3 | 20 April 2018 | 13:43 | 10 | 5 | 50.1 | 25 | 21.4 | 40.9 | NE 3–7 |
| UAS, Smoke 4 | 20 April 2018 | 15:07 | 10 | 5 | 50 | 25 | 23.0 | 37.8 | NE 3–10 |
| UAS, Smoke 5 | 20 April 2018 | 15:32 | 10 | 5 | 50 | 25 | 25.4 | 33.6 | NE 1–3 |
| UAS, Smoke 6 | 20 April 2018 | 16:08 | 10 | 5 | 50.5 | 25 | * | * | NE 1–3 |
| Ground, Background 1 | 20 April 2018 | 11:15 | 10 | 5 | 50.83 | 2 | 20.3 | 44.1 | NE 3–7 |
| UAS, Background 1 | 20 April 2018 | 12:31 | 10 | 5 | 49.8 | 25 | 19.5 | 44.6 | NE 3–7 |
| Ground, Background 2 | 20 April 2018 | 11:35 | 10 | 5 | 50 | 2 | 21.5 | 42.2 | NE 3–7 |
| UAS, Background 2 | 20 April 2018 | 13:04 | 10 | 5 | 50.1 | 25 | 20.8 | 41.6 | NE 3–7 |
| Sample Type | UAS, Smoke 1 | UAS, Smoke 2 | UAS, Smoke 3 | UAS, Smoke 4 | UAS, Smoke 5 | UAS, Smoke 6 | Ground, Background 1 | UAS, Background 1 | Ground, Background 2 | UAS, Background 2 |
|---|---|---|---|---|---|---|---|---|---|---|
| PM 1 | 250 | * | 371 | 316 | 39 | * | 16 | 14 | 21 | 18 |
| PM 2.5 | 520 | * | 950 | 582 | 55 | * | 21 | 18 | 31 | 23 |
| PM 10 | 559 | * | 1021 | 618 | 67 | * | 23 | 19 | 33 | 23 |
| Total CFU m−3 | 2750 | 1224 | 1463 | 202 | 1136 | 596 | 159 | 1944 | 888 | 1273 |
| Cladosporium sp. 1 | x | x | x | x | x | x | x | x | x | x |
| Altenaria alternata | x | x | ||||||||
| Cladosporium sp. 2 | x | |||||||||
| Unk fungi 2 | x | |||||||||
| Unk fungi 3 | x | x | ||||||||
| Unk bacteria 1 | x | |||||||||
| Paenibacillus sp. | x | x | x | |||||||
| Nocardia sp. | x | x | ||||||||
| Unk Fungi 1 | x | x | x | x | ||||||
| Unk fungi 4 | x | x | ||||||||
| Non-Sporulating Fungi | x | x | ||||||||
| Epicoccum nigrum | x | x | x | |||||||
| Penicillium sp. | x | x | x | x | ||||||
| Bacillus sp. | x | x | x | x | ||||||
| Unk bacteria 2 | x | |||||||||
| Lysinbacillus sp. | x | |||||||||
| Sporolactobacillus sp. | x |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kobziar, L.N.; Pingree, M.R.A.; Watts, A.C.; Nelson, K.N.; Dreaden, T.J.; Ridout, M. Accessing the Life in Smoke: A New Application of Unmanned Aircraft Systems (UAS) to Sample Wildland Fire Bioaerosol Emissions and Their Environment. Fire 2019, 2, 56. https://doi.org/10.3390/fire2040056
Kobziar LN, Pingree MRA, Watts AC, Nelson KN, Dreaden TJ, Ridout M. Accessing the Life in Smoke: A New Application of Unmanned Aircraft Systems (UAS) to Sample Wildland Fire Bioaerosol Emissions and Their Environment. Fire. 2019; 2(4):56. https://doi.org/10.3390/fire2040056
Chicago/Turabian StyleKobziar, Leda N., Melissa R. A. Pingree, Adam C. Watts, Kellen N. Nelson, Tyler J. Dreaden, and Mary Ridout. 2019. "Accessing the Life in Smoke: A New Application of Unmanned Aircraft Systems (UAS) to Sample Wildland Fire Bioaerosol Emissions and Their Environment" Fire 2, no. 4: 56. https://doi.org/10.3390/fire2040056