Abstract
Many metals form passive oxides which are broken or weakened by anionic-induced degradation leading to material failure and anionic diffusion is an important step of this degradation process. The diffusion of anionic species through oxides involves a combination of diffusion along grain boundaries, cracks, and channels, as well as bulk diffusion via point defects which is the focus of this study. Using density functional theory, we study bulk diffusion of Cl through O vacancies in α-Cr2O3 as a model system for passive metal oxides. Little is known about Cl diffusion and bonding characteristics, so we benchmark our work through comparison to numerous studies on O diffusion in α-Cr2O3 to analyze similarities and differences between the O and Cl diffusion in the passivation and degradation process of α-Cr2O3 respectively. Unlike O diffusion, the lowest diffusion barrier for Cl is cross-plane diffusion between two (0001) planes through a vacant cation site but the much shorter in-plane diffusion path within the same coordination polyhedron has 35% higher barrier. This work provides the basis for considering the contributions of Cl bulk-diffusion in the overall diffusion kinetics of Cl through α-Cr2O3.
Export citation and abstract BibTeX RIS
Corrosion is a major threat to the extended service life of structures resulting in millions of dollars in cost from replacements parts due to loss and failure. 1 Stainless steels are iron-based alloys, containing a maximum of 1 ± 2% carbon and a minimum of 10.5% chromium, widely used in civil infrastructure due to their excellent mechanical properties and corrosion resistance characteristics. 2 The corrosion resistance of stainless steel is due to a stable, durable, and self-repairing thin continuous oxide/hydroxide passive film that forms on the surface of the alloy. 2–4 Extensive studies, using X-ray photoelectron spectroscopy (XPS) and X-ray absorption near edge spectroscopy (XANES), found that the passive film of stainless steel formed in low pH aqueous environment is to a large extent enriched by chromium on the surface as α-Cr2O3 and Cr(OH)3. 4–9 The exposure of stainless steel components to aggressive ions such as chlorides (e.g. seawater) can however lead to the loss of passivity and a localized corrosion known as pitting. 10
Chlorides are known to affect the passivity of the stainless steels, mainly as a result of their ability to increase the dissolution rates of the metal. 11 Previous studies have shown a strong correlation between chloride concentrations in the electrolyte and corrosion potential of steel alloys, indicating that the presence, and amounts of, chlorides exposed to the alloy surface are critical to the degradation of the passive film. 12,13
It has been hypothesized that the diffusion of Cl into the passive film leads to the breakdown of the film, 10 however, the role of chlorides in the degradation of passive films on stainless steel is still debated. 14–20 There are two proposed models to describe the interaction of chlorides with the passive film, namely, the point defect model 21,22 and the ion exchange model. 10,16,23,24 The point defect model suggests a chloride-induced cation vacancies formation on the surface of the oxide film followed by a subsequent rupturing of the oxide film due to stress from the build-up of cation vacancies at the metal-film interface. 4–9 Unlike the point defect model, the ion exchange model proposes penetration of chloride into the passive film via oxygen vacancies or ion exchange with subsurface anions. 10,24 Experimental analysis of passive films 14–17 on chromium and chromium-rich alloys, such as stainless steel, have reported chlorides inside the passive film and at the metal/film interface in high chloride concentration. 25,26 These observations are consistent with the ion exchange model 24 which proposes that chlorides diffuse into the passive film, weakening the film or damaging the metal/film interface. The ion exchange model further proposes that the chloride causes active dissolution of the metal at the metal/film interface and subsequent breakdown by a different mechanism such as stress due to void formation and rupture of the film. 27–29 The presence of chlorides inside the oxide film is central to the ion exchange model and is supported by the observation of chlorides inside the passive films of: nickel using XPS and radiotracer measurements, 25 Fe–Cr–Ni alloy using aberration-corrected TEM (Cs-corrected TEM) and a fast and precise super X-ray energy-dispersive spectrometer (Super-X EDS) analysis 26 in high concentration chloride containing solutions as well as by computational studies. 30,31
Density functional theory (DFT) calculations can provide essential insights into the Cl interactions and mobility within the passive film. In our previous work, we showed that Cl insertion into the subsurface via oxygen vacancies near the surface is thermodynamically feasible at high Cl surface coverage (≥10/12 ML) and by exchange with subsurface anions at full coverage in support of the ion exchange model. 31 Insertion of Cl into an interstitial space in α-Cr2O3 was unfavorable in our calculations. The presence of chloride inside α-Cr2O3 passive oxide of stainless steel is supported by the experimental results of Saadi et al. 17 who found high chloride concentrations at the metal/film interface of 316 l stainless steel incorporated during film formation in NaCl. The insertion of Cl into the subsurface was also found to be thermodynamically feasible in NiO passive layer above 75% Cl coverage consistent with the ion exchange model. 30
Once subsurface, the Cl diffusion is predominantly through grain boundaries; 32,33 however, other diffusion mechanisms, including diffusion through oxygen vacancies (bulk diffusion) and short-circuit diffusion (by micro-cracks, porosities, or channels), should also be considered. 34,35 The hypothesis for Cl diffusion through oxygen vacancies is based on the presence of oxygen vacancies in the passive film during the passivation process at the metal/film interface. 21,36 Oxygen vacancies also dominate the point defects that remain on the surface and in the bulk of the passive film after passivation and are possible pathways for Cl diffusion in the bulk. 37 Several DFT investigations have focused on bulk diffusion of O in oxides to predict their activation energies for diffusion and the most favorable diffusion pathways. 38–42 The bulk diffusion of Cr and O in α-Cr2O3 is an important step in the kinetics of the oxide growth on stainless steel and other Cr alloys and understanding of which has been advanced both computationally 38–41,43–46 and experimentally. 47–49 Despite the importance of Cl in oxide degradation, 17,24,50 Cl diffusion in chromium oxide films is much less studied. Zhang et al. 26 studied Cl diffusion in amorphous and nanocrystalline Fe3O4 using DFT and found that Cl diffusion via O vacancy had the lowest energy barrier at the amorphous and nanocrystalline interfaces. We are not aware of any studies of Cl diffusion in α-Cr2O3.
Here we use DFT to investigate the bulk diffusion of Cl along the (0001) plane (in-plane), and across the (0001) plane (cross-plane) of α-Cr2O3, as a surrogate for passive film on stainless steels 4,8 to help elucidate the mechanism of Cl diffusion through an α-Cr2O3 passive layer.
In this paper, we report the activation barriers for Cl diffusion between two oxygen vacancies along the same plane and across two planes of α-Cr2O3. The presence of O vacancies in bulk may facilitate the insertion and diffusion of Cl into the bulk to take up excess electrons available at the vacant O site. Unlike oxygen diffusion, the lowest diffusion barrier for Cl diffusion is diffusion between two (0001) planes rather than along the (0001) plane. The low barrier is due to high coordination of the Cl at the transition state. This suggests that Cl diffusion differs from the much more studied oxygen diffusion.
Methods
Computational details
The calculations herein are based on the spin-polarized DFT, as implemented in the Vienna Ab Initio Simulation Package (VASP), 51–53 using generalized gradient approximation (GGA) with the Perdue-Burke-Erzerhof (PBE) exchange correlation functional. 54 Electron-ion interactions were described using projector augmented wave (PAW) method and a plane wave cut-off energy of 520 eV for all calculations. 55,56 The Methfessel–Paxton smearing method 57 was used for all calculations. The effects of strong intra-atomic electronic correlations between the Cr 3d5-orbitals were modeled by adding an on-site Coulomb repulsion, Hubbard term (U), according to the DFT + U method. 58,59 A U-J = 4 eV was applied based on our previous studies and the lattice parameters calculated for α-Cr2O3 using the DFT + U are a = 5.06 Å and c = 13.92 Å. 31
A (2 × 2 × 2) supercell is used for all the calculations to determine the relative feasibility of different diffusion pathways, 38–41,60 but the supercell size has been shown to affect barrier heights due to high O vacancy concentration in a periodic system. 42 The minimum energy paths of Cl diffusion were determined using the climbing image nudged elastic band (CI-NEB) method for five images including the initial and the final states. 61 A 3 × 3 × 1 Monkhorst-Pack k-point sampling was used for all calculations, and ground state configurations and CI-NEB bands were minimized to less than 0.01 eV Å−1 total forces on each ion.
Models
The α-Cr2O3 bulk is an antiferromagnetic oxide with the corundum structure and space group R  60,62
Figure 1(a) shows the α-Cr2O3 unit cell comprised of a hexagonal closed-packed O matrix where two-thirds of the octahedral sites are occupied by Cr cations and one-third are vacant. An O anion in the crystal structure is enclosed by four nearest neighboring Cr cations forming a four-fold (tetrahedral) coordination. The Cr ions in the α-Cr2O3 have an antiferromagnetic spin configuration below 307 K
63,64
and a magnetic structure corresponding to + - + - spin sequence along the [111] axis and overall zero magnetic moment for the unit cell.
64,65
This magnetic configuration was adopted for our calculations.
60,62
Figure 1(a) shows the α-Cr2O3 unit cell comprised of a hexagonal closed-packed O matrix where two-thirds of the octahedral sites are occupied by Cr cations and one-third are vacant. An O anion in the crystal structure is enclosed by four nearest neighboring Cr cations forming a four-fold (tetrahedral) coordination. The Cr ions in the α-Cr2O3 have an antiferromagnetic spin configuration below 307 K
63,64
and a magnetic structure corresponding to + - + - spin sequence along the [111] axis and overall zero magnetic moment for the unit cell.
64,65
This magnetic configuration was adopted for our calculations.
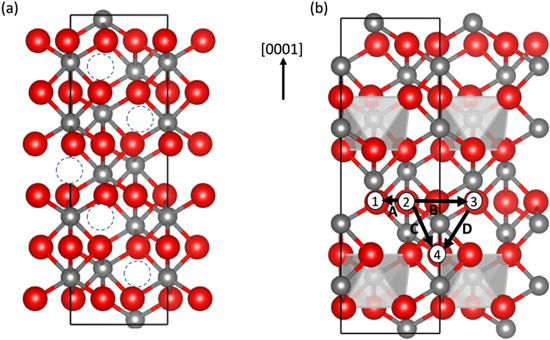
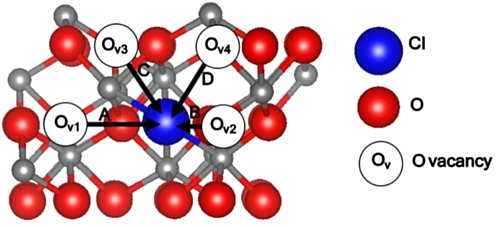
Figure 1. Atomistic structures presenting (a) α-Cr2O3 unit cell where unoccupied octahedral sites are shown in dashed circles. (b) Map of different Cl diffusion pathways in the α-Cr2O3 lattice, in- plane paths A(2 → 1) and B(2 → 3), and cross-plane paths C(2 → 4) and D(3 → 4) and the outlines of the coordination polyhedrons. Cr and O are represented in grey and red spheres respectively.
Download figure:
Standard image High-resolution imageFigure 1b shows the Cl diffusion paths between O sites in the α-Cr2O3 unit cell. The paths are categorized into two types, in-plane and cross-plane diffusion paths with reference to the [0001] direction of the α-Cr2O3 unit cell in Fig. 1a. The in-plane Cl diffusion involves diffusion along the (0001) plane (x-y direction in Fig. 1) along the shortest O–O distance within the coordination polyhedron, as in path A, or between two coordination polyhedrons along the longest O–O distance, as in path B in Fig. 1b. The cross-plane Cl diffusion is between two oxygen layers in the neighboring (0001) planes (z-direction in Fig. 1). Two main trajectories are identified for Cl diffusion across the neighboring (0001) planes (z-direction ): a cross-plane diffusion between two Cr cations, as in path C (Fig. 1b), and a cross-plane diffusion through a vacant octahedral site in the O sublattice, shown as path D in Fig. 1b.
Results and discussion
Diffusion paths and barriers
Figure 2 shows the minimum energy pathway for the two in-plane and the two cross-plane Cl diffusion paths. All the minimum energy paths studied appear to be nearly are symmetric with one transition state.
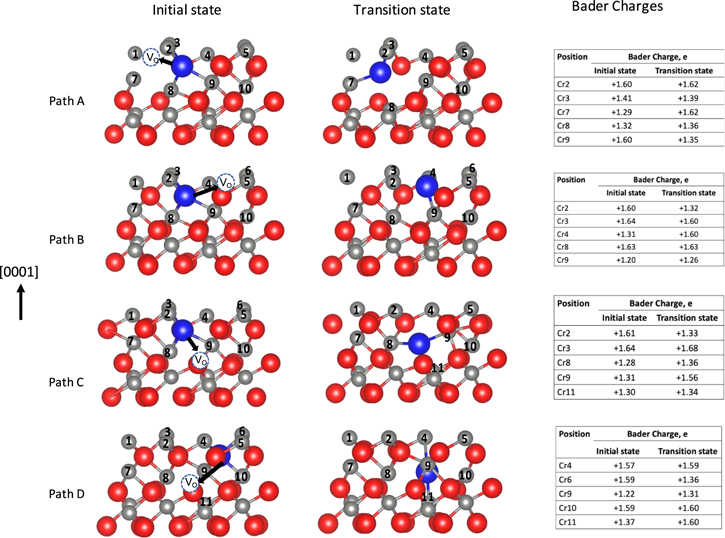
Figure 2. Comparison of the potential energy (left) for Cl diffusion along different diffusion paths(right). Red, blue and grey spheres represent O, Cl, and Cr respectively. The dashed circles represent initial positions of the O vacancy. Note: Only a portion of the entire calculated cell in the vicinity of the Cl and vacancy bulk defects is shown. The dot markers on the potential energy plot indicate the images included in the NEB calculations.
Download figure:
Standard image High-resolution imageThe Cl diffusion in paths A and B are in-plane. The initial and final states for path A are within the same coordination polyhedron while the final state for path B is in an adjacent polyhedron. For in-plane diffusion, the Cl diffusion to an O vacancy within the same coordination polyhedron (path A) is more favorable than to an adjacent polyhedron (path B). This is consistent with previous DFT studies 33,38,40,41,45,66 of O diffusion via O vacancy in α-Cr2O3 which showed that the diffusion of O along the (0001) plane is most favorable within the same coordination polyhedron.
The cross-plane diffusion paths, C and D, are shown in Fig. 2. The cross-plane diffusion of Cl through an unoccupied O interstice (path D) has a lower activation energy than Cl diffusion through a Cr bilayer (path C). Similar observations were made by Gray et al. 39 for O diffusion across the (0001) plane to a neighboring O vacancy, where the most favorable path traverses a vacant O interstice.
Path D has the overall lowest activation barrier of the paths studied. The second lowest energy path is the in-plane diffusion within the coordination polyhedron (path A) which has 35% higher activation barrier than the lowest barrier (path D). This suggests that Cl diffusion in α-Cr2O3 would be fastest through neighboring O vacancies across the (0001) plane direction passing through an unoccupied Cr site, followed by diffusion between O vacancies within the coordination polyhedron along the (0001) plane. This differs from the lowest energy diffusion path for O diffusion in α-Cr2O3 reported previously 39,40,45 where the in-plane diffusion of O within the coordination polyhedron (comparable diffusion route to path A herein) has the lowest energy. This may be due to the different coordination for the O and Cl species at the transition state, discussed in the next section.
Bader analysis, chemical bonding
Bader charge analysis of the initial and transition states was used to study the charge transfer from the Cr and the interactions between the Cr and Cl along the diffusion path, the findings of which are summarized in Fig. 3, where Cr lattice sites have been labeled Cr1 through Cr10. A Cr–Cl bond is shown for bond distances ≤ 2.55 Å, which is slightly longer than the Cr–Cl bond distance in a CrCl3 molecule which is 2.4 Å. 67
Figure 3. Bader charges for initial and transition state structures of diffusion paths A-D. The Bader charges of the Cr atoms and their corresponding position numbers in the initial and transition state structures are shown in the tables. Cr, O, and Cl are represented with grey, red, and blue spheres, respectively. Only a portion of the entire calculated cell in the vicinity of the Cl and vacancy bulk defects is shown.
Download figure:
Standard image High-resolution imageCr has an octahedral coordination in an α-Cr2O3 crystal, forming six Cr–O bonds with an average bond distance of ∼2.03 Å and Bader charges of +1.68 e and −1.11 e on Cr and O, respectively. Cr ions are partially reduced in the presence of a neighboring O vacancy 68 and thus expected to have Bader charge ≪ +1.68 e as in Cr7 of the initial state of path A, which has a charge of +1.29 (Fig. 3a). The Cr–Cl bond is weaker than the Cr–O bond so when Cl occupies an O vacancy, charge transfer to the Cl from the nearest neighboring Cr atom is smaller. For example, Cr2 in the initial state of path A, has a charge of +1.60 e as it donates less electrons to the Cl than it would to O. In cases where the Cr interacts with both Cl and an O vacancy, the Cr ion is expected to have a charge value between the charge of Cr near an O vacancy and a Cr that is interacting with a Cl. Cr ion of this bonding characteristics have a charge ranging from ∼+1.20 to ∼+1.56.
The initial state of paths A-C have four Cr–Cl bonds (with Cr2, Cr3, Cr8, and Cr9) and four Cr-vacancy interactions (Cr1, Cr3, Cr7, and Cr8). Path A has two Cr–Cl bonds at the transition state (Cr3 and Cr7), which is attained by breaking three out of the four Cr–Cl bonds formed at the initial state (involving Cr2, Cr8, and Cr9) and formation of a new bond with Cr7 within the same polyhedron. Path B also has two Cr–Cl bonds (involving Cr4 and Cr9) at the transition state and four Cr–Cl bonds (involving Cr4, Cr6, Cr9, and Cr10) at the final state in adjoint polyhedron. Unlike path A, the new Cr–Cl bond formed at the transition state in path B involves a Cr (Cr4) from the adjoining coordination polyhedron.
Path C also has two Cr–Cl bonds (Cr8 and Cr9) at the transition state attained by breaking two Cr–Cl bonds (involving Cr2 and Cr3). Path D has four Cr–Cl bonds (Cr4, Cr6, Cr9, and Cr10) at the initial state but three Cr–Cl bonds (Cr4, Cr9, and Cr11) at the transition state unlike paths A–C which have two Cr–Cl bonds. The transition state of path D is achieved by breaking two Cr–Cl bonds (involving Cr6 and Cr10) and forming one Cr–Cl bond (involving Cr11). The high coordination of the Cl at the transition state for path D, stabilizes the transition state and lowers the activation energy.
For Cl diffusion, we find the Cl to have the highest (three-fold) coordination at the transition state in path Din contrast with the results of Gray et al. 39 for O diffusion in α-Cr2O3 which found O to have a four-fold coordination at the transition state for path A. This may contribute to the relatively low diffusion barrier of O for path A relative to Cl.
To our knowledge, an experimental determination of the diffusivity of Cl in chromia has not been reported to date, but oxygen diffusion in chromia has been measured in single crystals 48 and grain boundaries 69–71 using O18 tracers. The self-diffusion of isotopic oxygen in α-Cr2O3 determined for equilibrium temperatures 1100o to 1450o C reported an activation energy of 4.731 ± 0.069 eV. 69 A direct comparison between our activation barriers and the experimentally-determined energy barrier is difficult because the latter also includes the barrier to oxygen vacancy formation or a neutral defect such as a neutral O/Cr pair. Medasani et al. 45 reported a 2.3 eV barrier for vacancy-mediated self-diffusion of O in α-Cr2O3 based on DFT calculations which is more than double the lowest Cl diffusion barrier reported herein. This is due to the high electronegativity of O leading to stronger Cr–O than the Cr–Cl bond and more stable initial state for the O diffusion. This suggests a faster vacancy-mediated diffusion of Cl in α-Cr2O3 relative to O although other processes such as oxygen vacancy formation may play a large role in the overall diffusion process. The oxygen vacancy formation energy has been calculated by previous studies to be 4.11 eV 44 and 5.11 eV. 40 The large vacancy formation energies suggest a high barrier to the formation of oxygen vacancies in a perfect α-Cr2O3 bulk after the film is fully formed. The Cl diffusion via oxygen vacancies may therefore be especially favored during the film formation where oxygen vacancies are available in relatively large amounts, being generated at the metal/film interface. 21 This is consistent with experimental observations of the presence of chlorides inside the film and at the metal/film interface of stainless steel formed in chloride-containing solutions while no and very small amounts of chlorides in passive film formed in air, 16,21 and film formed in low pH solution and then exposed to chlorides respectively. 14,26
Previous studies have reported grain boundary diffusion being the main pathway for species (Cr, O) diffusion in bulk α-Cr2O3. 35,70,71 Lei et al. 34 compared the vacancy-mediated O diffusion in α-Al2O3 using DFT and reported a lower activation energy for O diffusion (1.26 eV) along the Σ3(0001) grain boundaries compared to bulk diffusion of O (3.58 eV). These studies suggest that grain boundary diffusion is also likely to play an important role in Cl diffusion in α-Cr2O3.
The effect of defects on the density of states of α-Cr2O3 bulk
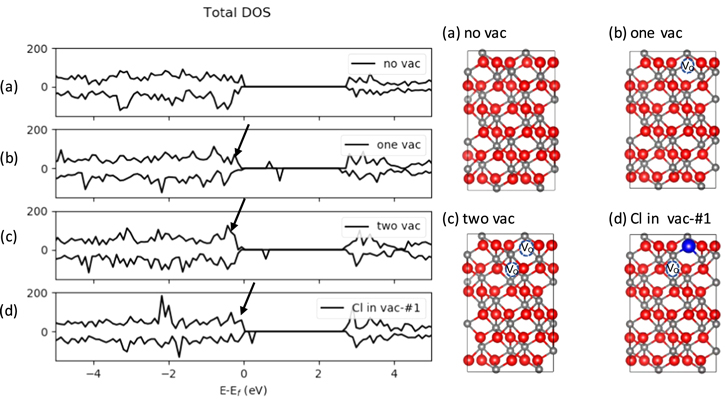
To understand the effect of the presence of the defect structures necessary for lattice diffusion of Cl on the electronic structure of α-Cr2O3, the densities of states (DOS) for four different structures are compared: a defect free bulk (no vac), bulk with one or two O vacancies per simulations cell (one or two vac), and bulk where one out of two O vacancies (denoted by vac-#1) is occupied by Cl (Cl in vac-#1). The configuration for the Cl in vac-#1 is the same as the initial configuration of Path D, the diffusion path with the lowest energy barrier described in a previous section. The different α-Cr2O3 bulk structures and their density of states (DOS) are shown in Fig. 4. The calculated bandgap between the valence band maximum (VBM) and the conduction band minimum for α-Cr2O3 bulk is 2.86 eV, which is an underestimation compared to experimental value but comparable to previous DFT + U values. 39,40,59 The creation of an O vacancy introduces two unoccupied spin up and spin down states in the band gap at roughly 0.8 eV and 1 eV respectively, above the Fermi level in agreement with previous studies of α-Cr2O3 showing that, the presence of O/Cr vacancies in the bulk introduces defect states in the bandgap. 39,41 The O vacancy reduces the Cr ions in prior contact with the now vacant O site leading to electron gain by the Cr ions shown by larger DOS near the Fermi level (indicated by the arrow in Fig. 4b). The vacant O site, referred to as the F-center by Hayes and Stoneman, 72 is a neutral vacancy possessing two electrons which can be available to the surrounding metal ions or to the diffusing Cl in this case. The introduction of two O vacancies across the plane of a Cr–O polyhedron however leads to a small partially occupied defect state (see Fig. 4c) and an unoccupied defect state in the bandgap at roughly 0.8 eV. There is also an increase in the DOS near the Fermi level (indicated by arrow in Fig. 4c) beyond what was seen for the single vacancy defective bulk, suggesting a more reduced bulk with increasing number of O vacancies. However, this is expected to increase the energy of O vacancy formation as metal-oxygen bonds become stronger as a result of the gain in electrons by the metal ions. The defect state shifts to just slightly above the Fermi level (E-Ef = 0) when one of the vacancies is occupied by Cl (see Fig. 4d) as the other defect state closest to the valence band disappears.
Figure 4. Total DOS of α-Cr2O3 bulk crystal for a perfect structure (a, no vac), a defective structure with one O vacancy per a (2 × 2) cell (b, one vac), a defective structure with two O vacancies per a (2 × 2) cell in the same polyhedron (c, two vac), and defective structure with one Cl and one O vacancy per a (2 × 2) cell across the plane of a coordination polyhedron (d, Cl in vac- #1). Cr, Cl and O are represented in grey, blue, and red spheres respectively. Arrows indicate DOS near the Fermi level.
Download figure:
Standard image High-resolution imageThis is an indication of the strong interactions of the Cl with the O vacancy as the Cl draws electrons from the nearest neighboring Cr ions which have been reduced by the creation of the vacancies. This suggests that the presence of O vacancies may facilitate the insertion and diffusion of Cl into the bulk to take up excess electrons available at the F-centers resulting from the creation of the vacancies.
Conclusion
We used density functional theory to study the diffusion of Cl in α-Cr2O3, a critical step of Cl-induced degradation of passive films according to the ion exchange model. The most favorable Cl diffusion path is cross-plane diffusion where the Cl is stabilized by three Cr at the transition state. The diffusion between neighboring O vacancy within the coordination polyhedron is the most favorable in-plane diffusion path where the Cl is stabilized by two Cr at the transition state. Comparison of our findings with previous calculations of O diffusion in α-Cr2O3 suggests that vacancy-mediated Cl diffusion in α-Cr2O3 is higher than O. Like O anions, Cl forms tetrahedral coordination with Cr ions in the lattice and reduces the number of defect states introduced by the O vacancies in the band gap. These results provide essential insights into Cl permeation through the passive layer in their onward journey to the metal/film interface as proposed by experimental studies.
Acknowledgments
The research is supported by the U.S. Department of Energy, Nuclear Energy University programs under the grant DE-NE0008668. Part of the calculations used the Extreme Science and Engineering Discovery Environment (XSEDE) 73 (allocations TG-DMR160093 and TG-ENG170002) which is supported by National Science Foundation grant number ACI-1053575. The authors acknowledge the Texas Advanced Computing Center (TACC) at the University of Texas at Austin and the Comet cluster at the San Diego Supercomputer Center, and the High-Performance Computing (HPC) center at Idaho National Laboratory for providing HPC resources that have contributed to the research results reported within this paper. KOS acknowledges the Idaho National Laboratory summer internship program.