Abstract
The Asian citrus psyllid (ACP), Diaphorina citri, was detected for the first time in the Republic of Benin, West Africa. The ACP is a known vector of Candidatus Liberibacter asiaticus (CLas), the putative causal agent of the devastating Huanglongbing (HLB; citrus greening disease). During visual surveys, ACP was only observed on residential citrus trees in southern Benin, but not in residential areas or commercial groves in the central and northern parts of the country. Its identity was confirmed morphologically and molecularly via DNA barcoding with published primers. Analysis of the obtained sequences showed that the ACP recorded in Benin clustered with the ones previously reported from Nigeria, suggesting a common origin of both populations. The ACP samples from Benin also carried Ca. Carsonella ruddii and Ca. Profftella armatura, two commonly found ACP endosymbionts. However, all the sampled ACP individuals tested negative for Ca. Liberibacter africanus, Ca. Liberibacter americanus, and CLas by quantitative polymerase chain reaction. This is the second report of the ACP in West Africa after Nigeria, the eastern bordering country of the Republic of Benin. Benin has an expanding commercial citrus industry, especially in the southern part of the country. Although the ACP samples tested negative for the HLB associated bacteria, the detection of ACP in the country requires swift actions including area-wide surveys to determine the extent of spread of this pest and the implementation of eradication or control efforts to prevent its establishment and spread of HLB in the country.
Similar content being viewed by others
Introduction
In the Republic of Benin, citrus is grown commercially as a cash crop and as backyard tree like in many other parts of the world. Commercial citrus production in the country started soon after the independence in the 1960s, with the creation of the national fruits and vegetables company (Société Nationale des Fruits and Légumes, SoNaFeL) via a Benin-Israeli cooperation1. From 1960 to the 1970s, several citrus varieties of lime, lemon, grapefruit, mandarin, sweet oranges and tangelo were introduced for the establishment of commercial groves mainly in the southern part of the country2,3,4,5, but sweet orange remained the dominant commercial citrus species in the Republic of Benin to date6.
From the onset of commercial citrus industry in Benin, the planted acreage slowly increased and reached ca. 2500 ha of sweet oranges in 1977 where it plateaued until 1989. Commercial citrus acreage declined from 1989 to 1992, as a consequence of the closure in 1986 of SoNaFeL because the country underwent the World Bank and International Monetary Fund structural adjustment programs7. Subsequently, the harvested commercial orange acreage has rebounded and gradually increased reaching ca. 6500 ha in 20206. Despite the apparent expansion in acreage in recent years mainly due to the implantation of a processing plant in the commercial citrus belt of Benin, citrus yields in Benin remain among the lowest in the world. In 2020, average orange yields were estimated at 2.5 MT ha−1, the second lowest commercial yields recorded in Africa and far below the world average of 14–15 MT ha−16. Several biotic and abiotic factors are responsible for these meager citrus yields in Benin. Firstly, commercial citrus groves in Benin have no irrigation systems and trees are only rain fed. Erratic rainfall can negatively affect citrus yields and production8,9, as citrus trees require adequate water supply for fruit sizing. In addition, citrus groves in Benin remain largely unmanaged due to poor access to inputs including fertilizers and agrochemicals10. Under these conditions, pests and diseases can develop unchecked leading to substantial damage and poor yields. Indeed, over 80% of commercial citrus growers in Benin list the lack of agricultural inputs and pressure from pests and diseases as major constraints to citrus production, with arthropod pests and diseases reported as the most important impediments10. The hot and humid tropical climate prevailing in the citrus production areas of Benin favor the establishment and spread of many arthropod pests and diseases. Among the arthropod pests limiting citrus productivity in Benin, aphids (Aphis gossypii Glover and Myzus persicae (Sulzer)), the citrus leafminer (Phyllocnistis citrella Stainton), the grasshopper Zonocerus variegatus (Linnaeus), the green tree ant Oecophylla smaragdina (Fabricius), the cottony cushion scale Icerya purchasi Maskel, the citrus rust mite Phyllocoptruta oleivora (Ashmead) and fruit piercing moths (Othreis fullonia (Clerk), Ophideres sp., Achaeasp sp. and Enmonodia sp.) are cited as of great concern2,3,7,11,12,13. Zadji14 reported a complex of termites (Trinervitermes occidentalis (Sjöstedt), Amitermes guineensis (Sjöstedt), Macrotermes bellicosus (Smeathman), and Ancistrotermes crucifer (Sjöstedt) as the most devastating group of arthropod pests, boring galleries into stems, thus weakening trees and reducing their productivity by 25–50%. Vayssieres et al.12 listed dipteran fruit flies (Bactrocera. invadens Drew, Tsuruta & White, B. cucurbitae, Ceratitis fasciventris (Bezzi), C. ditissima (Munro), C. anonae Graham, and Dacus punctatifrons Karsch), as pests of significant importance causing substantial losses of fruit quality and about 35% loss of total production in sweet orange varieties.
In addition to direct feeding damage, some arthropod pests are recognized vectors of pathogens causing economically damaging citrus diseases. One such pathogen, the citrus tristeza virus (CTV), is the most destructive virus known to citrus production15, and its efficient vector the brown citrus aphid (Toxoptera aurantii (Boyer de Fonscolombe)) is endemic in Benin5,13. Despite the economic importance of CTV in citriculture, Huanglongbing (HLB) or citrus greening is currently the most devastating citrus disease worldwide16. HLB is a destructive disease that has spread into major citrus production areas in the Americas and Africa16,17,18,19. HLB is putatively caused by three related phloem-inhabiting fastidious bacteria namely Candidatus Liberibacter africanus (CLaf), Ca. Liberibacter americanus (CLam) and Ca. Liberibacter asiaticus (CLas)16. These bacteria are spread via vegetative propagation of infected plant materials and by the two psyllid vectors, the African citrus triozid (ACT, Trioza erytreae (Del Guercio) (Hemiptera: Triozidae)) and the Asian citrus psyllid (ACP, Diaphorina citri Kuwayama (Hemiptera: Liviidae)). While ACT is known to specifically transmit CLaf restricted to the African continent, CLam and CLas are spread by ACP20. CLam is known to occur exclusively in the Americas, but CLas has spread across continents, currently occurring in major citrus producing countries in Africa, America and Asia19,21,22,23.
Diaphorina citri is an invasive and fast spreading pest. During the past few decades, ACP has invaded several countries and states in the Americas22,24,25 and Africa21,26,27,28. ACP presence in sub-Saharan Africa represents a significant threat to the sustainability of citrus production in these countries, which is already crippled by several other biotic and abiotic production constraints. Although the presence of CLas in Africa is currently limited to East Africa21,26,27, the detection of ACP in Nigeria in West Africa28 points to a continent-wide potential of spread via trade and natural dispersal of this highly prolific vector.
Like ACP, ACT is an invasive species that is the main vector of CLaf, but has been shown to experimentally transmit CLas. Although ACT mainly occurs in eastern and southern Africa and the Middle East in Asia29, ACT has invaded Portugal and Spain in Europe in recent years30,31. However, its presence in West Africa is not fully elucidated.
Improving citrus yields and production will require mitigating the impacts of the many biotic factors currently identified as the most important production constraints. Given the presence of ACP in neighboring Nigeria28, and the high propensity of both ACP and ACT to spread and colonize new areas, studies are needed to determine their occurrence in the citrus producing areas of Benin. An early detection of ACP and ACT in an area is critical for the enactment of effective mitigation efforts. The presence of both psyllids in Benin has not been previously evaluated. This study was conducted to determine whether ACP and ACT and the HLB pathogens CLas and CLaf occur in residential and commercial citrus locales in Benin.
Materials and methods
Sample collection
A citrus pest survey was conducted at six residential sites and three commercial groves in December 2021 in five of the 12 departments of the Republic of Benin to assess the presence of D. citri and T. erytreae (Table 1). The residential sites were visited upon homeowners’ approval to evaluate the health of trees because of either poor growth or the presence of sooty mold. The commercial groves located in close proximity of the main highway were surveyed and sampled during a road trip. At each site, the host plant species was determined by evaluated the leaves and/or fruit when present, and the tree or grove location coordinates were recorded using the Compass app of an IPhone 12 pro32. All residential host plants present at the survey sites were visually examined for the presence of arthropod pests with special reference to the life stages of ACP or their wax-like feeding droppings on leaves33 and of ACT and the small pit galls it causes on young leaves34. In commercial groves, a sample of ten trees was randomly selected for evaluation. As young flush shoots are the breeding sites for ACP and ACT34,35, these flush shoots when present, were preferentially examined. Suspect ACP nymphs and adults were collected and stored in plastic vials containing 95% ethanol until further analysis and processing. No ACT was collected during the surveys, hence subsequent analyses focused on ACP.
Identification of D. citri
Morphological identification
All insect samples were brought to the Texas A&M University-Kingsville Citrus Center (TAMUK-CC) entomology laboratory for further analysis. Using the morphological characteristics described by Mead33 and Yang36, the identification of suspect nymphs and adults was made by comparing them to archived voucher specimens kept in the laboratory. Voucher specimens of identified D. citri from Benin are kept at TAMUK-CC.
Nucleic acid isolation and PCR
Total nucleic acids were extracted from morphologically identified D. citri adults and nymphs following the Dellaporta et al.37 protocol. A NanoDrop 2000 series spectrophotometer (Thermo Fisher Scientific Inc., Waltham, MA, USA) was used to quantify and analyze the quality of the extracted nucleic acids. After quantification and quality analysis, the total nucleic acid samples were stored at − 20 °C until when they were subjected to polymerase chain reaction (PCR) assays as described by Oke et al.28. Briefly, a 2 µL aliquot of each total nucleic acid sample was used as template in a 25 μL PCR with reagents and Rapid Protocol described for the PrimeSTAR GXLDNA Polymerase (Takara Bio USA, Inc., Mountain View, CA). PCR was performed on each insect total nucleic acid sample using three distinct primer pairs that target specific genes encoded by the ACP and two of its endosymbionts. The 834 bp fragment of the mtCOI coding region was targeted by the DCITRI COI-L (5′-AGGAGGTGGAGACCCAATCT-3′) and DCITRI COI-R (5′-TCAATTGGGGGAGAGTTTTG-3′) primer pair38. Additionally, single copy housekeeping gene-specific primer pairs argH-F1 (5′-CTCCTATGCCTGGATTTACTCA-3′) & argH-R1 (5′-TTGATTAGGCGCTGTACCTCC-3′) and atpA-F1 (5′-CAATAATCGGTATCGCTGTT-3′) & atpA-R1 (5′-AGCATATTACGGAAGGTGAT-3′) were used to target the argH of the psyllid primary endosymbiont (P-endosymbiont) Ca Carsonella ruddii and the atpA of the secondary endosymbiont (S-endosymbiont) Ca Profftella armatura, respectively20. The DNA amplicons from these samples were ran on 1% agarose gels pre-stained with ethidium bromide along with the 100–2000 bp Wide-Range DNA Ladder (Takara Bio USA, Inc.) and visualized under a UV-transilluminator. For all analyses, DNA extracts from laboratory colony of D. citri maintained at TAMUK-CC were included as positive controls.
Cloning and sequencing
Cloning and sequencing of DNA samples were conducted as described by Oke et al.28. Succinctly, target specific DNA bands of the correct sizes of the D. citri samples were excised and gel-eluted using the Zymoclean™ Gel DNA Recovery Kit (Zymo Research, Irvine, CA). Using the CloneJET PCR Cloning Kit (Thermo Fisher Scientific), the recovered DNA samples were ligated individually into the pJET1.2/blunt vector as per the manufacturer’s recommendations. Chemically competent DH5α Escherichia coli cells were transformed from the ligation products, and two to three plasmids with PCR-verified correct size inserts per cloned DNA amplicon were isolated from recombinant E. coli cells using the GenElute Plasmid Miniprep Kit (Sigma-Aldrich, St. Louis, MO). Each plasmid sample was sequenced in both directions with the pJET1.2F (5′-CGACTCACTATAGGGAGAGCGGC-3′) and pJET1.2R (5′-AAGAACATCGATTTTCCATGGCAG-3′) primers by the Sanger method in a commercial facility (ELIM BIOPHARM, Hayward, CA, USA).
Bioinformatic analysis
The raw sequences were trimmed to remove the pJET1.2 flanking multiple cloning site sequences with the VecScreen (https://www.ncbi.nlm.nih.gov/tools/vecscreen/). A consensus sequence from each of the sample-specific forward and reverse sequences was derived using the CAP contig assembly program of the BioEdit software39. The derived sequences were scanned with the National Center for Biotechnology Information (NCBI) GenBank database using the BLASTN program40 for species identification purposes. The MUSCLE alignment program41 was used to perform multiple sequence alignments for gene-specific datasets of sequences derived from samples analyzed in this study and corresponding sequences of taxon representative retrieved from GenBank. The gene-specific alignment files were used to determine the sequence identity matrices and for phylogenetic analysis with the maximum likelihood algorithm of the molecular evolutionary genetics analysis (MEGA) software version 7.042.
Testing for the presence of Ca. Liberibacter spp.
DNA extracts from all the ACP samples collected in Benin were assayed for the presence of CLaf, CLam and CLas using the Taqman multiplex real-time PCR assays as reported by Oke et al.28. Known positive and negative control DNA samples, and non-template water control were included in the reactions. All samples with a cycle threshold (Ct) ≤ 37 were considered positive for a specific target bacterium.
Results
ACP detection and morphological identification
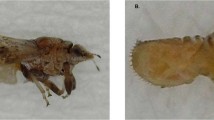
Nine locations (residential sites = 6 and commercial groves = 3) were surveyed during the study (Table 1). Five major ACP host plants including grapefruit, lime, orange jasmine, sweet orange and tangerine were identified at the various locations. Suspect ACP samples were only collected at three residential sites in southern Benin, and none was found in commercial groves and in residential sites in the central and northern departments. The comparison of morphological features of collected samples to voucher specimens33,36,43 led to their identification as D. citri (Fig. 1). ACP was recovered from three host plants including lime, orange jasmine and sweet orange during the study. While adults were collected from three sites, nymphs were only found on sweet orange trees at one location (Table 1). Considering the spatial separation (2–10 km) of the three detection sites in southern Benin, D. citri may likely be established in this part of the country. During the surveys, aphids and soft scales, including the brown soft scale and the cottony cushion scale, were the most dominant pest species found infesting trees, and they are very likely responsible for sooty mold occurring on the trees.
The lateral (A) and ventral (B) views of adult Asian citrus psyllid (DiaphorinacitriKuwayama) and corresponding dorsal (C) and ventral (D) views of D. citrinymphs sampled from different locations (Table 1) in southern Republic of Benin.
Molecular detection
A representative subset of 10 randomly collected ACP (4 adults and 6 nymphs) from the three positive detection sites was selected for molecular analysis. Gene-specific DNA amplicons of the expected sizes were obtained from these samples. Ten mtCOI-specific (GenBank accession no. OP414612–OP414621), 8 argH-specific (OP414446–OP414453) and 10 atpA-specific (OP414454–OP414463) sequences were derived. Analysis of these sequences using BLASTN produced highly significant matches (≥ 99% nt identity; 100% query coverage; E-value 0.0) to corresponding gene-specific sequences of D. citri, Ca. Carsonella ruddii and Ca. Profftella armatura, respectively available in GenBank. In pairwise comparisons, the mtCOI sequences derived in this study shared 99–100% nt identities among themselves and the same range of nt identities with corresponding global sequences of D. citri, indicating that they belong to this psyllid species. As expected, the derived mtCOI sequences from the Benin insect samples were significantly distinct from (and shared only 42–43% identity with) the corresponding sequences of T. erytreae isolates retrieved from GenBank. Based on pairwise comparisons, the argH- and atpA-specific sequences derived in this study shared ~ 100% nt identity with their respective gene-specific sequences and 97–100% and 99.5–99.8% nt identity with corresponding global sequences of Ca. Carsonella ruddii (P-endosymbiont) and Ca. Profftella armatura (S-endosymbiont), respectively. The results provide definitive confirmation of the identity of the Benin suspect individuals as D. citri and showed that they carry the same primary and secondary bacterial endosymbionts in their bacteriome as previously documented in ACP from other citrus-producing areas of the world20,44,45.
Phylogenetic analysis
The reconstructed mtCOI maximum likelihood (ML) phylogenetic trees showed the clustering of sequences from Benin within the D. citri clade and distinct from the T. erytreae clade (Fig. 2A). When the ML tree was reconstructed with only D. citri mtCOI sequences, they clearly segregated into the previously defined Western and Eastern clades20 with strong (> 50%) bootstrap support (Fig. 2B). All the adult and nymph mtCOI sequences from Benin (OP414612 to OP414621) clustered into the Western clade (Fig. 2B). The argH-specific sequences of the P-endosymbiont Ca. Carsonella ruddii also segregated into the previously defined Western and Eastern clades46 with strong (> 60%) bootstrap support (Fig. 2C, Supplementary table). Like the ACP mtCOI sequences, all the argH sequences from Benin (n = 8; GenBank acc. Nos. OP414446–OP414453) also clustered into the Western clade (Fig. 2C). In contrast to the mtCOI and argH sequences, the atpA sequences of the S-endosymbiont Ca. Profftella armatura segregated into three distinct clades with strong (> 60%) bootstrap support (Fig. 2D, Supplementary table). Interestingly, all the atpA sequences from Benin (n = 10; GenBank acc. Nos. OP414454–OP414463) segregated into the previously defined ‘African’ clade, distinct from the Western and Eastern clades (Fig. 2D). This ‘African’ clade was derived from atpA gene of Ca. Proftella armatura detected in ACP collected in Nigeria28. Taken together, the results showed that the field-collected psyllids in Benin and their bacterial endosymbionts are genetically uniform and belonged to the species Diaphorina citri. All the analyzed psyllid samples were negative for CLaf, CLam and CLas by qPCR.
Maximum Likelihood (ML) phylogenetic trees depicting the evolutionary relationships between adults and nymphs of the Asian citrus psyllid (Diaphorina citri Kuwayama), and their primary and secondary endosymbionts, sampled from different locations (Table 1) in southern Republic of Benin, West Africa and the corresponding sequences of global populations of each taxon. The ML trees were derived based on analyses of sequences specific to the mtCOI gene of D. citri (A and B): OP414612 to OP414621 derived in this study and others from GenBank; the argH gene of the primary endosymbiont Ca. Carsonella ruddii (C): OP414446 to OP414453 derived in this study and others from GenBank; and the atpA gene of the secondary endosymbiont Ca. Profftella armatura (D): OP414454 to OP414463 derived in this study and others from GenBank. The sequences derived in this study are shaded in black color. The Tamura 3-parameter was determined as the model with the lowest BIC (Bayesian Information Criterion) scores and was therefore used in ML phylogenetic analysis for each of the gene-specific sequences (with 1000 bootstrap replications). Branches with < 60% bootstrap support were collapsed.
Discussion
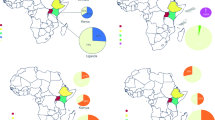
Using both morphological identification and molecular tools, our study confirmed the suspect specimens collected in Benin to be D. citri. While D. citri has long been recognized as an invasive pest22, this is to the best of our knowledge of current literature, the first report of its detection in Benin. The presence of ACP on the African continent was previously reported from countries in East Africa including Tanzania21, Kenya26 and Ethiopia27, and in Nigeria in West Africa28 (Fig. 3). The current detection in Benin shows that the geographic range of ACP is expanding on the continent, possibly through the increased trade among countries, movement of citrus transplants by hobbyist growers, or natural dispersal. However, its presence in only three residential sites in the south suggests that its spread may be currently limited in the country. Surprisingly, the ACP was detected feeding on three of the most suitable host plants47,48 in the coastal part of the country with altitude less than 20 m above sea level (Table 1). It is well established that higher altitude limits the incidence of the ACP with its population levels decreasing at higher altitudes probably as a result of differential temperature, air pressure, oxygen level, ultraviolet light or their combinations49. However, all sampling sites were below 600 m above sea level, the cut-off point above which no ACP was collected in Puerto Rio49. The limited distribution of the ACP in Benin opens avenues for effective management and the enactment of strict quarantine regulations to prevent the incursion of D. citri into the commercial citrus groves mostly located in southern and central parts of the country. The prevailing hot and humid conditions in Benin with temperature ranging from 28 to 32 °C all year round, indicate that the whole country is suitable for the establishment and development of D. citri. Indeed, using a temperature-based model of suitability, Taylor et al.50 reported that Africa has climate suitable for the establishment of D. citri and HLB. Considering the biology and ecology of D. citri, Aidoo et al.51 used Maximum Entropy (MaxEnt) to predict that all citrus production areas in Africa are suitable for its establishment although the actual suitability varies with regions. Under current climatic conditions, Benin lies from the high suitability area in the southern and central parts of the country to the medium suitability area in the north51, indicating that the whole country is at high risk of D. citri and HLB invasion. The commercial citrus belt is located in the southern part of the country that is deemed highly suitable for D. citri reproduction and development and only situated ~ 100 km away from the current detection sites. This close proximity of the commercial citrus production area to the current D. citri detection sites represents a grave menace to the sustainability of citriculture in Benin. Citrus production in Benin is already crippled by a myriad of biotic and abiotic constraints and a lack of agrochemical inputs. The invasion of D. citri and possible HLB introduction will further reduce yields that are already among the lowest in the world6. Hence, a rapid response plan needs to be implemented at the national level, and perhaps regionally across West Africa, to limit the spread of this invasive pest and to control its population within currently established areas.
Map of Africa showing current incidence of the Candidatus Liberibacterasiaticus (CLas) and its Asian citrus psyllid (ACP) vector. The approximate locations where the ACP was detected in Nigeria (Oke et al.28) and the Republic of Benin (this study) are shown in the enlarged map of West Africa.
To gain more insights into the biodiversity of this quarantine significant pest, we analyzed the mitochondrial cytochrome oxidase (mtCOI) gene of specimens collected in Benin. Not only that the mtCOI is versatile because of its high prevalence in cells relative to nuclear genes, it is a highly conserved and maternally inherited gene that is independent of life stages, polymorphism and gender52,53. The mtCOI gene has been used with great success in previous D. citri population studies20,28,38,54,55. Similarly, the gene-specific sequence of the primary (Ca. Carsonella ruddii) and secondary (Ca. Profftella armatura) endosymbionts of D. citri were used to evaluate their diversity. All the analyzed D. citri individuals from Benin and their primary endosymbiont (Ca. Carsonella ruddii) formed a homogenous group and clustered within the Western clade that consists of psyllid populations that generally include individuals from Asia and the Americas46,56,57. However, all the atpA sequences from Benin segregated into the previously defined ‘African’ clade derived from D. citri collected from neighboring Nigeria, and distinct from the Western and Eastern clades28. These results highly suggest that the D. citri samples collected from Benin and Nigeria may be of the same origin and given the close proximity of both countries (Fig. 3), it is highly plausible that this pest may have spread from one country to another. Moreover, these results points to the fact that D. citri may also have been present for quite some time in Benin for local adaptation of the secondary endosymbiont to occur as previously hypothesized28. Such local adaptation of Ca. Profftella armatura may be warranted due to its role as a defensive endosymbiont against natural enemies in insects45.
The presence of sooty mold on foliage and fruit of the surveyed host plants was the precursor of this study. Sooty mold is generally a cosmetic problem on fruit, but its heavy presence as observed on some leaves can impair photosynthesis, leading to poor tree growth and productivity. This is because the black coating of the sooty mold fungi on leaves could intercept sunlight and perturb leaf temperature and transpiration, subsequently affecting the water balance of trees58. Although, the ACP was found at one location where sooty mold was recorded on plants, the main culprits of this symptom were soft scales (brown soft scale and cottony cushion scale), aphids and whiteflies that were abundant throughout the country, indicative of poor pest management in citrus production in Benin. In addition, at that site both ACP nymphs and adults were recorded, probably because of the presence of young shoots on sweet orange trees (Table 1). The ACP is known to reproduce exclusively on young shoots35 that were absent in most locations at the time of sampling.
Increasing citrus production in Benin will require inputs of agrochemicals, irrigation, and effective locally-adapted integrated pest and disease management (IPDM). The detection of D. citri in Benin poses another significant challenge for the sustainability of citrus production, especially if D. citri were to acquire Candidatus Liberibacter asiaticus, causal agent of HLB. Insights gleaned from other invaded citrus producing areas of the world indicate that HLB detection always (and most often surely) lag D. citri detection18,19,59. One major foundation for the development of an effective IPDM program will be an understanding of the identity and population dynamics of pests and disease cycles in commercial citrus groves. With the worldwide spread of invasive species, area-wide surveys need to be frequently conducted for early detection of any introduced pest and disease and subsequent implementation of eradication or mitigation approaches.
This work represents a limited and quick study to assess the presence of psyllid vectors of HLB in Benin. The positive detection of D. citri following that of neighboring Nigeria in 202028 calls for extensive and regional field surveys to determine the extent of its spread across West Africa and especially in the commercial citrus production areas. Although all the D. citri samples analyzed in this study and those from Oke et al.28 tested negative for the HLB bacterium, it will be important to continue to systematically survey, sample, and test D. citri from the study sites and surrounding areas since CLas detection in psyllids often predates detection in trees59. In conclusion, the current restricted distribution of D. citri in Benin offers an opportunity for limiting its spread and effectively controlling its populations (“Supplemantary information”).
Data availability
The datasets generated and/or analyzed during this study are available in the GenBank. For the mtCOI-specific (GenBank accession no. OP414612–OP414621). SUB12050645 BenNy1-1 OP414612; SUB12050645 BenNy1-2 OP414613; SUB12050645 BenNy2-8 OP414614; SUB12050645 BenNy2-9 OP414615; SUB12050645 BenNy3-12 OP414616; SUB12050645 BenNy3-13OP414617; SUB12050645 BenAd1-17 OP414618; SUB12050645 BenAd1-18 OP414619; SUB12050645 BenAd2-22 OP414620; SUB12050645 BenAd2-24 OP414621. A copy of the files can be viewed at: https://nam04.safelinks.protection.outlook.com/?url=https%3A%2F%2Fsubmit.ncbi.nlm.nih.gov%2Fsubs%2F%3Fsearch%3DSUB12050645&data=05%7C01%7CMamoudou.Setamou%40tamuk.edu%7Cbbbb36a7a1d147a8554a08da9d82ea60%7C17420fd64d7546859adf7a650964dbcf%7C0%7C0%7C637995482851818680%7CUnknown%7CTWFpbGZsb3d8eyJWIjoiMC4wLjAwMDAiLCJQIjoiV2luMzIiLCJBTiI6Ik1haWwiLCJXVCI6Mn0%3D%7C3000%7C%7C%7C&sdata=q96cGgwGIfL%2FU68uEYMON25c%2FxBXUQUH%2Ft3wD2qHQUY%3D&reserved=0. For the argH-specific (OP414446 to OP414453) and the 10 atpA-specific (OP414454 to OP414463) and also herein provided as supplemental materials.
References
Afloukou, F., Zinsou, V. & Onelge, N. Citrus in Benin Republic: Past, present, and future challenges. Citrus Res. Technol. 41, ee1060. https://doi.org/10.4322/crt.20820 (2020).
Praloran, J. C. L’agrumiculture Dahoméenne. Situation actuelle, amélioration et développement (GERDATIFAC, Paris, 1972).
Montagut, G. Note sur les variétés d’agrumes importées au Dahomey (GERDAT-IFAC, 1974).
Vogel, R. Compte-rendu de mission au Dahomey du 16 au 21 janvier 1972: Problèmes phytosanitaires posés à l’agrumiculture dahoméenne (GERDAT-IFAC/INRA, San Giuliano, 1972).
Vogel, R. Compte-rendu de mission au Dahomey du 19 au 25 avril 1975: Etat phytosanitaire des agrumes dahoméens (GERDAT-IFAC/INRA, San Giuliano, 1975).
Food and Agriculture Organization of the United Nations—FAO. Food and Agriculture Organization of the United Nations database (FAOSTAT). Rome: FAO. Retrieved 12 Aug 2022 from http://www.fao.org/faostat/ (2022).
Lokossou, B., Tossou, C., Varnier, C. & Ollitrault, P. Mission d’évaluation de l’agrumiculture au Bénin (INRAB, Cotonou, 2009).
Dorji, K., Lakey, L., Chophel, S., Dorji, S. D. & Tamang, B. Adoption of improved citrus orchard management practices: A micro study from Drujegang growers, Dagana, Bhutan. Agric. Food Secur. 5, 1–8 (2016).
Qin, W., Assinck, F. B. T., Heinen, M. & Oenema, O. Water and nitrogen use efficiencies in citrus production: A meta-analysis. Agric. Ecosyst. Environ. 222, 103–111 (2016).
Akohoue, F., Segnon, A. C. & Achigan-Dako, E. G. Diversity in smallholder citrus orchards and cultivation bottlenecks: Research avenues for improved production in Benin, West-Africa. Exp. Agric. 54, 641–654. https://doi.org/10.1017/S001447971700028X (2018).
Atachi, P., Desmidts, M. & Durnez, C. Les papillons piqueurs (Lépidoptères, Noctuidea) ravageurs des agrumes au Bénin: Dégâts qu’ils occasionnent et caractéristiques morphologiques. FAO Plant Prot. Bull. 37, 111–120 (1989).
Vayssieres, J., Adandonon, A., Sinzogan, A. & Korie, S. Diversity of fruit fly species (Diptera: Tephritidae) associated with citrus crops (Rutaceae) in southern Benin in 2008–2009. Int. J. Biol. Chem. Sci. 4, 1881–1897. https://doi.org/10.4314/ijbcs.v4i6.64966 (2011).
Massokonon, M. J. B. Impact de l’implantation de l’usine de transformation d’orange en jus d’orange sur la production et la commercialisation d’orange à Za-Kpota: Etude diagnostique (Mémoire de Licence). Faculté des Sciences Agronomiques, Université d’Abomey-Calavi, Abomey-Calavi, République du Bénin (2015).
Zadji, L. D. V. Entomopathogenic nematodes as potential control agents of termites in citrus in Benin (Ph.D. thesis). University of Ghent, Belgium (2014).
Lee, R. F. Control of virus diseases of citrus. Adv. Virus Res. 91, 143–173. https://doi.org/10.1016/bs.aivir.2014.10.002 (2015).
Bové, J. M. Huanglongbing: A destructive, newly emerging, century-old disease of citrus. J. Plant Pathol. 88, 427–453 (2006).
da Graça, J. V. Citrus greening disease. Annu. Rev. Phytopathol. 29, 109–136. https://doi.org/10.1146/annurev.py.29.090191.000545 (1991).
Gottwald, T. R. Current epidemiological understanding of citrus Huanglongbing. Annu. Rev. Phytopathol. 48, 119–139. https://doi.org/10.1146/annurev-phyto-073009-114418 (2010).
da Graça, J. V. et al. Huanglongbing: An overview of a complex pathosystem ravaging the world’s citrus. J. Integr. Plant Biol. 58, 373–387 (2016).
Wang, Y. et al. Genetic diversity of Diaphorina citri and its endosymbionts across east and south-east Asia. Pest Manag. Sci. 73, 2090–2099 (2017).
Shimwela, M. M. et al. First occurrence of Diaphorina citri in East Africa, characterization of the Ca. Liberibacter species causing Huanglongbing (HLB) in Tanzania, and potential further spread of D. citri and HLB in Africa and Europe. Eur. J. Plant Pathol. 146, 346–368. https://doi.org/10.1007/s10658-016-0921-y (2016).
Grafton-Cardwell, E. E., Stelinski, L. L. & Stansly, P. A. Biology and management of Asian citrus psyllid, vector of huanglongbing pathogens. Annu. Rev. Entomol. 58, 413–432 (2013).
Saponari, M. et al. First report of Candidatus Liberibacter asiaticus associated with Huanglongbing in sweet Orange in Ethiopia. Plant Dis. 94, 482. https://doi.org/10.1094/pdis-94-4-0482a (2010).
French, J. V., Kahlke, C. J. & da Graça, J. V. First record of the Asian citrus psylla Diaphorina citri Kuwayama (Homoptera:Psyllidae), in Texas. Subtrop. Plant Sci. 53, 14–15 (2001).
Yamamoto, P. T., Paiva, P. E. B. & Gravena, S. Population dynamics of Diaphorina citri Kuwayama (Hemiptera: Psyllidae) in citrus orchards in the North of Sao Paulo State, Brazil. Neotrop. Entomol. 30, 165–170 (2001).
Rwomushana, I. et al. Detection of Diaphorina citri Kuwayama (Hemiptera: Liviidae) in Kenya and potential implication for the spread of Huanglongbing disease in East Africa. Biol. Invasions 19(10), 2777–2787. https://doi.org/10.1007/s10530-017-1502-5 (2017).
Ajene, I. J. et al. Detection of Asian citrus psyllid (Hemiptera: Psyllidae) in Ethiopia: A new haplotype and its implication to the proliferation of Huanglongbing. J. Econ. Entomol. 113, 1640–1647. https://doi.org/10.1093/jee/toaa113 (2020).
Oke, A. O., Oladigbolu, A. A., Kunta, M., Alabi, O. J. & Sétamou, M. First report of the occurrence of Asian citrus psyllid Diaphorina citri (Hemiptera: Liviidae), an invasive species in Nigeria, West Africa. Sci. Rep. 10, 1–8 (2020).
EPPO. EPPO Global database. In EPPO Global database, Paris, France: EPPO. https://gd.eppo.int/ (2022).
Otero, R. P., Vázquez, J. P. M. & Del Estal, P. Detección de la psila africana de los cítricos, Trioza erytreae (Del Guercio, 1918) (Hemiptera: Psylloidea: Triozidae), en la Península Ibérica. Arq. Entomol. 13, 119–122 (2015).
Hernández, A. G. Trioza erytreae (Del Guercio 1918): Nueva plaga de los citricos en Canarias. Phytoma España Rev. Prof. Sanid. Veg. 153, 112–118 (2003).
Apple Inc. Compass Navigation (Version 3.4) [Mobile application software]. Retrieved from https://www.apple.com/app-store/ (2020).
Mead, F. W. The Asiatic citrus psyllid, Diaphorina citri Kuwayama (Homoptera: Psyllidae). Florida Department of Agriculture Conservation Service, Division of Plant Industry Entomol Circular No. 180. http://www.freshfromflorida.com/pi/enpp/ento/entcirc/ent180.pdf (1997).
Aidoo, O. F. et al. Host suitability and feeding preference of the African citrus triozid Trioza erytreae Del Guercio (Hemiptera: Triozidae), natural vector of “Candidatus Liberibacter africanus”. J. Appl. Entomol. 143, 262–270. https://doi.org/10.1111/jen.12581 (2019).
Sétamou, M., Flores, D., French, J. V. & Hall, D. G. Dispersion patterns and sampling plans for Diaphorina citri (Hemiptera: Psyllidae) in citrus. J. Econ. Entomol. 101, 1470–1487 (2008).
Yang, C. T. Psyllidae of Taiwan. Taiwan Mus. Special Publ. Ser. 3, 37–41 (1984).
Dellaporta, S. L., Wood, J. & Hicks, J. B. A plant DNA mini preparation: Version II. Plant Mol. Biol. Rep. 1, 19–21 (1983).
Boykin, L. M. et al. Overview of worldwide diversity of Diaphorina citri Kuwayama mitochondrial cytochrome oxidase 1 haplotypes: Two old world lineages and a new world invasion. Bull. Entomol. Res. 102, 573–582 (2012).
Hall, T. A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acid Symp. 41, 95–98 (1999).
Altschul, S. F., Gish, W., Miller, W., Myers, E. W. & Lipman, D. J. Basic local alignment search tool. J. Mol. Biol. 215, 403–410 (1990).
Edgar, R. C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucl. Acids Res. 32, 1792–1797 (2004).
Kumar, S., Stecher, G. & Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 33, 1870–1874 (2016).
EPPO. EPPO Standards PM 7/52(1). Diagnostic protocol for Diaphorina citri. Bull. OEPP EPPO Bull. 35, 331–333. https://doi.org/10.1111/j.1365-2338.2005.00839.x (2005).
Thao, M. L., Clark, M. A., Burckhardt, D. H., Moran, N. A. & Baumann, P. Phylogenetic analysis of vertically transmitted psyllid endosymbionts (Candidatus Carsonella ruddii) based on atpAGD and rpoC: Comparisons with 16S–23S rDNA-derived phylogeny. Curr. Microbiol. 42, 419–421 (2001).
Nakabachi, A. et al. Defensive bacteriome symbiont with a drastically reduced. Curr. Biol. 23, 1478–1484 (2013).
Wang, Y. et al. Phylogeography of Diaphorina citri (Hemiptera: Liviidae) and its primary endosymbiont, ‘Candidatus Carsonella ruddii’: An evolutionary approach to host-endosymbiont interaction. Pest Manag. Sci. 74, 2185–2194 (2018).
Sétamou, M., da Graça, J. V. & Sandoval, J. L. II. Suitability of native North American Rutaceae to serve as hosts for the Asian citrus psyllid (Hemiptera: Liviidae). J. Appl. Entomol. 140, 645–654 (2016).
Meng, L., Cheng, X., Xia, C. & Zhang, H. Effect of host plants on development and reproduction of Diaphorina citri and their host preference. Entomol. Exp. Appl. 170(8), 700–707. https://doi.org/10.1111/eea.13188 (2022).
Jenkins, D. A., Hall, D. G. & Goenaga, R. Diaphorina citri (Hemiptera: Liviidae) abundance in Puerto Rico declines with elevation. J. Econ. Entomol. 108, 252–258 (2015).
Taylor, R. A. et al. Predicting the fundamental thermal niche of crop pests and diseases in a changing world: A case study on citrus greening. J. Appl. Ecol. 56, 2057–2068. https://doi.org/10.1111/1365-2664.13455 (2018).
Aidoo, O. F. et al. A machine learning algorithm-based approach (MaxEnt) for predicting invasive potential of Trioza erytreae on a global scale. Ecol. Inform. https://doi.org/10.1016/j.ecoinf.2022.101792 (2022).
Simon, C. et al. Evolution, weighting, and phylogenetic utility of mitochondrial sequences and a compilation of conserved polymerase chain reaction primers. Ann. Entomol. Soc. Am. 87, 651–701 (1994).
Asokan, R. et al. COI based molecular identification of mango leaf hoppers (Hemiptera: Cicadellidae) in India. Indian J. Biotechnol. 14, 260–263 (2015).
de León, J. H. et al. Two separate introductions of Asian citrus psyllid populations found in the American continents. Ann. Entomol. Soc. Am. 104, 1392–1398 (2011).
Guidolin, A. S., Fresia, P. & Cônsoli, F. L. The genetic structure of an invasive pest, the Asian citrus psyllid Diaphorina citri (Hemiptera: Liviidae). PLoS ONE 9, e115749 (2014).
Saha, S. et al. Survey of endosymbionts in the Diaphorina citri metagenome and assembly of a Wolbachia wDi draft genome. PLoS ONE 7, e50067 (2012).
Lashkari, M. et al. Global genetic variation in the Asian citrus psyllid, Diaphorina citri (Hemiptera: Liviidae) and the endosymbiont Wolbachia: links between Iran and the USA detected. Pest Manag. Sci. 70, 1033–1040 (2014).
Insausti, P. E. L., Ploschuk, M., Izaguirre, M. & Podworny, M. The effect of sunlight interception by sooty mold on chlorophyll content and photosynthesis in orange leaves (Citrus sinensis L.). Eur. J. Plant Pathol. 143, 559–565 (2015).
Sétamou, M., Alabi, O. J., Kunta, M., Dale, J. & da Graça, J. V. Distribution of Candidatus Liberibacter asiaticus in citrus and the Asian citrus psyllid in Texas over a decade. Plant Dis. https://doi.org/10.1094/PDIS-08-19-1779-RE (2020).
Acknowledgements
We acknowledge all homeowners and growers who allowed us to survey citrus trees in their properties in Benin. We express our sincere thanks to Marissa Gonzalez and Ceasar Medelez (TAMUK Citrus Center) and Cecilia Villegas (Texas AgriLife Extension) for their assistance with molecular testing of the psyllid samples.
Author information
Authors and Affiliations
Contributions
M.S. and O.J.A. contributed to the study conception and design. Data collection and analysis were performed by M.S., O.J.A. and Y.L.S. All authors collectively wrote the manuscript. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sétamou, M., Soto, Y.L., Tachin, M. et al. Report on the first detection of Asian citrus psyllid Diaphorina citri Kuwayama (Hemiptera: Liviidae) in the Republic of Benin, West Africa. Sci Rep 13, 801 (2023). https://doi.org/10.1038/s41598-023-28030-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-28030-3
This article is cited by
-
Modelling the potential distribution of the Asian citrus psyllid Diaphorina citri (Hemiptera: Liviidae) using CLIMEX
International Journal of Tropical Insect Science (2024)
-
Addition of Selected Plant-Derived Semiochemicals to Yellow Sticky Traps Does Not Improve Citrus Psyllid Captures
Journal of Chemical Ecology (2024)
-
First report on the presence of huanglongbing vectors (Diaphorina citri and Trioza erytreae) in Ghana
Scientific Reports (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.