-
PDF
- Split View
-
Views
-
Cite
Cite
Lingjie Kong, Xue Chen, Ellen Heininger Yerger, Qiao Li, Fengxin Chen, Haiyun Xu, Fengjuan Zhang, Arbuscular mycorrhizal fungi enhance the growth of the exotic species Ambrosia artemisiifolia, Journal of Plant Ecology, Volume 15, Issue 3, June 2022, Pages 581–595, https://doi.org/10.1093/jpe/rtab087
Close - Share Icon Share
Abstract
Arbuscular mycorrhizal fungi (AMF) can increase host plant nutrient uptake via their mycelium, thus promoting plant growth. AMF have always been associated with successful invasion of most exotic plant species. However, knowledge regarding how AMF affect the success of plant invasion remains limited. Exotic Ambrosia artemisiifolia is an invasive and mycorrhizal plant species. A long-term field experiment was conducted to examine the differences in AMF diversity and composition in the roots of A. artemisiifolia and Setaria viridis subjected to interspecific competition during growth. A greenhouse experiment was also performed to test the effect of Funneliformis mosseae on the growth of these two species. Ambrosia artemisiifolia invasion caused AMF diversity to change in native S. viridis roots. Meanwhile, the relative abundance of F. mosseae was significantly higher in the roots of A. artemisiifolia than in those of S. viridis. The higher AMF colonization rate in the exotic species (A. artemisiifolia) than in the native species (S. viridis) was found in both the field and greenhouse experiments. The greenhouse experiment possibly provided that AMF advantaged to the growth of A. artemisiifolia, by influencing its photosynthetic capacity as well as its phosphorus and potassium absorption. These observations highlight the important relationship of AMF with the successful invasion of A. artemisiifolia.
摘要:
丛枝菌根真菌(AMF)可以通过其菌丝增加寄主植物对养分的吸收,从而促进植物生长。丛枝菌根真菌一直与大多数外来植物的成功入侵联系在一起。然而,有关丛枝菌根真菌如何影响植物入侵成功的机制仍然有待研究。豚草(Ambrosia artemisiifolia)是一种外来的菌根植物。通过长期田间实验,我们研究了种间竞争对豚草和狗尾草(Setaria viridis)根系丛枝菌根真菌多样性和组成的影响。此外,在温室实验中探究了摩西球囊霉(Funneliformis mosseae)对这两种植物生长的影响。研究结果表明,豚草入侵改变了本地植物狗尾草根系丛枝菌根真菌的多样性。另外,豚草根系中摩西球囊霉的相对多度显著高于狗尾草根系中。在田间和温室实验中均发现外来种豚草的丛枝菌根真菌侵染率高于本地种狗尾草。温室实验结果表明,丛枝菌根真菌通过影响豚草的光合能力以及磷和钾的吸收而促进豚草生长。这些研究结果揭示了丛枝菌根真菌和豚草成功入侵之间的重要关系。
INTRODUCTION
Arbuscular mycorrhizal fungi (AMF) can form mutualistic associations with exotic plant roots (Aslani et al. 2019; Lekberg et al. 2013). There is increasing evidence that the AMF community plays important roles in successful invasions of exotic plant species (Bunn et al. 2015; Hawkes et al. 2006; Zhang et al. 2018; Zubek et al. 2016). However, the related mechanism varies among these studies. Zhang et al. (2017) find that the high AMF colonization in exotic Flaveria bidentis that invaded China provides a competitive advantage over the native Setaria viridis. Lekberg et al. (2013) suggest that invasions by mycotrophic plants can increase AMF abundance and richness. Gomes et al. (2018) point out that the increased richness of AMF is associated with exotic plant host identity. Mummey and Rillig (2006) indicate that a significant reduction in AMF diversity occurs following the invasion of exotic species. However, a meta-analysis by Bunn et al. (2015) shows that colonization of AMF and their spore densities in exotic species are not significantly different from those associated with native plant species. Regardless of how the AMF diversity changes, an increasing amount of studies propose that associated AMF communities constitute an important component of the ecological strategies of exotic plants while invading a new habitat (Aslani et al. 2019; Callaway et al. 2008; Davison et al. 2020; Lekberg et al. 2013; Zubek et al. 2016). Thus, an improved understanding of the quantitative and qualitative differences in AMF communities between exotic and native species would be useful for predicting the ecological roles of AMF in the process of plant invasion.
The change in AMF abundance and diversity is often regarded as an important criterion in determining plant invasion success (Lekberg et al. 2013). Different AMF taxa have disparate effects on the competitive growth of plants because of differences in their respective abilities to obtain nutrients (Gioria and Osborne 2014; van der Heijden et al. 2003; Zhang et al. 2010). Exotic species may interact with different AMF taxa than their direct competitors and/or exploit specific beneficial interactions with mutualists to increase their competitiveness over that of competitors (Day et al. 2015; Moeller et al. 2015). However, several studies show that any difference in AMF community association between native and exotic species can affect the success of the invading plant (Stampe and Deahler 2003; Torrecillas et al. 2012), and some studies have identified AMF species that facilitate the invasion of exotic species. When an exotic plant invades a new habitat, the specific AMF that benefit the competitive growth of the exotic plant at a mechanistic level should be identified and studied.
Prior studies on the relationships between AMF and the invasion of exotic species primarily focus on the effects of plant invasions on AMF communities (Bunn et al. 2015; DeForest and Snell 2020; Gomes et al. 2018; Nuńez and Dickie 2014). However, only a few studies have examined the effects of AMF on the competitive growth between exotic and native species (Busby et al. 2013; Rodríguez-Caballero et al. 2018; Zhang et al. 2017, 2018). Moreover, the existing studies have reported different results regarding the effects of mycorrhizal fungi on plant invasion. Menzel et al. (2017) find that mycorrhizal fungi have a significant promoting effect on the successful invasion of exotic plants, whereas Rodríguez-Caballero et al. (2018) indicate that the unspecific relationships between the invasive Pennisetum setaceum and mycorrhizal fungi may provide advantages during its establishment. To further discover the role of AMF on the invasion of exotic species, more experiment studies with different plant species are required.
Our previous study shows that the invasion of Ambrosia artemisiifolia decreases the AMF diversity in its rhizosphere soil and that the AMF spores isolated from the rhizosphere soil of A. artemisiifolia increase the competitive ability of the invasive species (Zhang et al. 2018). These results indicate that mycorrhizal interactions between A. artemisiifolia and AMF play an important role in invasiveness of A. artemisiifolia. However, the particular species that facilitates its invasion has not been identified. To fill this gap in knowledge, it is necessary to examine the differences in AMF diversity and composition in the roots of both exotic and native plant species and to identify particular species that may provide A. artemisiifolia with a competitive advantage. We set up two experiments to examine the interspecific competition between A. artemisiifolia and S. viridis. The first was a long-term field experiment that examined AMF diversity in the roots of A. artemisiifolia and S. viridis growing under the conditions of interspecific competition, with the aim to study the quantitative and qualitative differences in the AMF communities of exotic and native plant species. We investigated whether the particular species hosted by A. artemisiifolia and S. viridis could provide more nutrients to A. artemisiifolia, facilitating its competitive growth. Therefore, a controlled pot experiment was conducted, with the aim of exploring specific beneficial interactions between A. artemisiifolia and mutualists that potentially enhanced the competitiveness of A. artemisiifolia over other plants. We hypothesized that AMF hosted by A. artemisiifolia may differ from those hosted by S. viridis, and the specific AMF may exert a positive effect on the growth of A. artemisiifolia yet a negative impact on S. viridis when both species were grown together. The results of the present study are expected to provide a broader understanding of the functional role of AMF in promoting the invasion of this exotic species.
MATERIALS AND METHODS
Field experiment: naturally occurring AMF communities in A. artemisiifolia and S. viridis roots
Experimental design
A long-term field experiment was established at the Langfang Experimental Station, Chinese Academy of Agricultural Science (CAAS), Beijing, China (39° 30′ 42ʺ N, 116° 36′ 07ʺ E). In 2016, mean annual rainfall was 555.1 mm and average temperature was 12.9 °C. The soil of the area was classified as a sandy clay loam Ferric Acrisol (Zhang et al. 2017). Soil physical and chemical properties were measured using the modified chromic acid titration method (Mc Lean and Watson 1985; Truog 1930; Walkley and Black 1934; Whitehead 1981) and they were listed as follows: 15.36 g kg–1 organic matter, 56.07 g kg–1 available nitrogen, 214.58 g kg–1 available potassium and 9.38 g kg–1 available phosphorus.
The exotic species A. artemisiifolia, a mycorrhizal species, belongs to the family Asteraceae and is widely distributed in China (Xu and Qiang 2004). Its invasion poses serious threats to local biodiversity and agricultural production (Ozaslan et al. 2016). In addition, this species, commonly known as ragweed, often causes human health problems due to its allergenic pollen (Ghiani et al. 2012). Ambrosia artemisiifolia is a mycorrhizal plant species. Setaria viridis (L.) Beauv. (Poaceae) is an annual C4 monocotyledon species native to China. It is often found in areas invaded by A. artemisiifolia.
Our experimental design was similar to that of a previous study (Zhang et al. 2018). The experimental plots (3 m × 2 m) were prepared in 2008, and each plot was provided with a 1-m isolation zone to prevent edge effects. Three treatments were used in the experiment: (i) A. artemisiifolia monoculture, (ii) an equal mixture of A. artemisiifolia and S. viridis (1:1) and (iii) S. viridis monoculture. Each treatment had five replicates. Before the seeds were sown, the plots were hand weeded to leave bare soil without tilling. The soil was watered to maintain soil field capacity at 40%. At the start of the experiment, 100 seeds that sterilized with 10% H2O2 were sown in each plot in May (for the mixture, we used 50 A. artemisiifolia seeds and 50 S. viridis seeds) (Zhang et al. 2017). No fertilizers were applied, and the composition of plant species in each plot was kept unchanged by manually removing the weeds every year. Each spring, all plants were pulled out of the soil, and the plots were reseeded to ensure that the target plants (A. artemisiifolia and S. viridis) would be exposed to the desired treatment conditions every year.
Five replicates were used for each treatment in 2008. After 8 years, the abundance of each plant species changed, and we reduced the number of replicates to three, in which the aerial cover of plants was similar. In 2016, the cover of S. viridis and A. artemisiifolia in the monocultures was 92% and 100%, respectively, whereas their cover in the mixture treatment was 20% and 75%. We followed the approach detailed by Huber and Ronchetti (2009) to identify the outliers of plants and thinned samples to obtain a sample that would robustly represent the mean. We visually estimated the mean and chose plants that seemed to be typical in each treatment. Five plants were selected per plant species and pooled together for subsequent index determination. Roots were collected from each treatment and species as follows: A. artemisiifolia roots in the A. artemisiifolia monoculture (A-mono), A. artemisiifolia roots in the mixture treatment (A-mix), S. viridis roots in the S. viridis monoculture (S-mono) and S. viridis roots in the mixture treatment (S-mix). The root cortex was carefully kept intact by manual collection, so that the AMF structures were retained for determination of their colonization rate and diversity. Plants roots were initially stored at 4 °C, and then at −80 °C after being washed with sterile dH2O.
Root colonization rates of AMF
AMF colonization rates in the A. artemisiifolia and S. viridis roots were analyzed using the magnified intersections method (Biermann and Linderman 1981; Giovannetti and Mosse 1980). From each replicate, 100 root segments of 1 cm were randomly selected for examination. AMF colonization was examined using an Olympus BX43 compound microscope (Olympus, Tokyo, Japan). In each root segment, colonization was regarded as successful if there were any fungal hyphae, vesicles or arbuscules. The scores of the 100 root segments were combined to calculate the colonization percentage.
DNA extraction from roots
One gram of each root sample was homogenized in liquid nitrogen. It was then mixed with 2 mL of the CTAB extraction buffer [2% (w/v) hexadecyl trimethyl ammonium bromide (CTAB), 1.4 mol L-1 NaCl, 0.02 mol L-1 EDTA [pH 8.0], 0.1 mol L-1 Tris-HCl [pH 8.0] and 0.2% (v/v) β-mercaptoethanol] preheated to 65 °C. The mixture was incubated at 65 °C for 1 h and centrifuged (−4 °C) at 13 500 rpm for 10 min after cooling. The supernatant was mixed with an equal volume mixture of phenol:chloroform:isoamyl alcohol mixture (25:24:1, v/v). After shaking, the mixture was centrifuged at 13 500 rpm for 10 min. Then, the supernatant was mixed with an equal volume mixture of chloroform:isoamyl alcohol mixture (24:1, v/v) and centrifuged twice at 12 000 rpm for 15 min. Afterwards, the upper phase was mixed with 0.1 volume of 3 mol L-1 NaAC and 0.6 volume of ice-cold isopropanol. DNA was precipitated at −20 °C for 3 h and centrifuged at 12 000 rpm for 10 min. The pellet was rinsed with 1000 mL ice-cold 75% ethanol, centrifuged at 10 000 rpm for 5 min, dried and dissolved in 50 mL of sterile dH2O. The DNA extracts were stored at −20 °C, and their quality and quantity were detected by 1% agarose gel electrophoresis.
Nested PCR analysis of AMF
We carried out two PCR amplifications of the small subunit rRNA genes. The first round of PCR was performed using universal fungal 18S rDNA primers Geo A2 (5′CCAGTAGTCATATGCTTGTCTC3′) and Geo 11 (5′ACCTTGTTACGACTTTTACTTCC3′) (Schwarzott and Schüßler 2001) to amplify the fungal 18S-specific sequence. The amplified fragment size was 1800 bp. PCR was performed in a final volume of 25 µL, which contained 2.5 µL 10× buffer, 1 µL template DNA, 0.5 µL dNTPs (10 mmol L−1), 0.5 µL GeoA2 (10 µmol L−1), 0.5 µL Geo11 (10 µmol L−1), 0.5 µL M5 Taq polymerase (5 U µL−1) and 19.5 µL ddH2O. Thermocycling conditions were as follows: initial denaturation at 95 °C for 5 min, followed by 35 cycles of 95 °C for 1 min, 55 °C for 1 min and 72 °C for 2 min, with a final extension at 72 °C for 10 min. The samples were then cooled to 4 °C. Control reactions contained no template DNA. Amplification products were detected by 1% agarose gel electrophoresis. The second PCR used the first PCR amplification product as a template. The second amplification was performed using forward primer NS31 (1 μL) with a mixture of equal amounts of the reverse AM1, AM2 and AM3 (AMmix, AM1, 5′GTTTCCCGTAAGGCGCCGAA3′; AM2, 5′GTTTCCCGTAAGGTGCCAAA3′; AM3, 5′GTTTCCC GTAAGGTGCCGAA3′; NS31, 5′TTGGAGGGCAAG TCTGGTGCC3′) to amplify the AMF SSU conserved region sequence (Santos-González et al. 2007). The amplicon length was 550 bp. The reaction mixture was the same as that in the first round of PCR. The temperature profile was programmed as follows: initial denaturation at 95 °C for 5 min, followed by 30 cycles of 95 °C for 45 s, 68 °C for 1 min and 72 °C for 90 s, and a final extension at 72 °C for 10 min. Then, the samples were cooled to 4 °C. Control reactions contained no template DNA. Amplification products were detected by 1% agarose gel electrophoresis, and they were purified and recycled with the EZgene Gel Extraction Kit 50 preps (Biomiga, San Diego, CA, USA) with a final elution volume of 50 µL.
Cloning, sequencing and phylogenetic analysis
Positive PCR products were inserted into the pEASY-T3 vector using the pEASY-T3 cloning kit (TransGen Biotech, Beijing, China) according to the manufacturer’s instructions. The products were transformed into competent Escherichia coli DH-5α. The transformed cells were then spread onto a Luria Bertani (LB) agar plate (50 mg mL−1 ampicillin, 24 mg mL−1 IPTG and 40 mg mL−1 X-gal) to identify white clones. After incubation overnight at 37 °C, the white clones were selected and transferred to LB broth, which contained 50 mg mL−1 ampicillin. After being incubated on a shaker-incubator (200 rpm, 37 °C) overnight, the putative positive transformants were reamplified with the specific primers NS31 and AM1. The temperature profile was programmed as: 8 min at 95 °C, 30 s at 95 °C, 30 s at 58 °C, 60 s at 72 °C for 30 cycles, 7 min at 72 °C, and cooling down to 4 °C. The product quality and size were detected on agarose gels as described above.
Each treatment had three replications, and approximately 50 positive clones were picked up in each replication. All the clones with inserts of the correct size were sequenced by General Biosystem Anhui Co., Ltd. (Anhui, China) in a single direction by using the universal primer M13F. The vector sequences were removed by MEGA software version 5 (Tamura et al. 2013). All PCR products were used to construct four SSU rDNA libraries. The sequences were submitted to NCBI for sequence similarity alignment. The representative sequences were aligned with other published AMF sequences, and neighbor-joining (NJ) phylogenetic analyses were performed using MEGA software version 5. The distances for the NJ tree were computed using default parameters. Scutellospora alterata was used as the outgroup. The rarefaction curves (with 95% confidence intervals) were constructed using PAST software version 3.11 (Øyvind Hammer, Natural History Museum, University of Oslo, Norway) to determine whether the sequencing depth was reasonable.
Diversity of the AMF community
The relative abundance of all AMF species in our total sample is calculated according to the following equation: Relative abundance = A / N × 100%, where A represents the number of sequences of one AMF sequence type and N represents the total number of sequences.
The impacts of invasive plants on the diversity of AMF in plant roots are assessed using the Shannon (H′) index, Simpson index (D) and Evenness index (J). These indices are calculated using the following equations, respectively (Zhang et al. 2017).
where S is the number of AMF species, Pi = ni/N, ni represents the number of AMF phylotype i and N is the total number of AMF phylotypes.
Greenhouse experiment: effect of AMF inoculum on interspecific competition between exotic and native plants
Experimental design
A greenhouse experiment was conducted at Hebei University (Baoding, China) to investigate how AMF influenced the competitive growth of A. artemisiifolia and S. viridis. The greenhouse experiment was a replacement design of that by Gibson et al. (1999). The growth substrate consisted of an autoclaved mixture (121 °C, 1 h, three times) of sandy clay and vermiculite (1:1 v/v). Plant seeds were sterilized in 10% H2O2 for 2 min, rinsed with sterile dH2O and sown in a pot (15 × 13 × 12 cm3). From the result of the field experiment, Funneliformis mosseae was one of the dominant AMF in the roots of A. artemisiifolia. Its relative abundance in the roots of A. artemisiifolia was significantly higher than in the roots of S. viridis. Therefore, we selected F. mosseae for the greenhouse experiment. This experiment included three treatments: (i) A. artemisiifolia monoculture, (ii) a mixture of A. artemisiifolia and S. viridis (1:1) and (iii) S. viridis monoculture. Four densities of F. mosseae spores (C0: 0, C1: 10, C2: 20 and C3: 30 spores g−1 soil) were selected based on the density of F. mosseae spores in the rhizosphere soil of A. artemisiifolia or S. viridis in the field. The inoculum was provided by Prof. Jinli Zhao (College of Life Science, Hebei University). The density of F. mosseae was measured using the wet-sieving and decanting technique for spores, as described in Zhao et al. (2001). Ten days after planting, the plants were thinned to four plants per pot (four plants of A. artemisiifolia or S. viridis in their monocultures and two plants of A. artemisiifolia with two plants of S. viridis in the mixture). The pots were placed in a climate chamber (25 °C for 24 h with a 14-h light and 10-h dark photoperiod) and arranged in randomized design. Each treatment was replicated eight times. Plants were watered every 2 days.
Measurements
Plants were harvested after 75 days. The roots and shoots from each treatment and species were collected separately. AMF colonization on the roots of A. artemisiifolia and S. viridis was determined using the magnified intersections method (Biermann and Linderman 1981; Giovannetti and Mosse 1980).
All roots and shoots were dried at 80 °C for 48 h. The plant total biomass and the corrected index for the relative competition intensity (CRCI) were calculated. CRCI was used to test the competitiveness of the investigated plants. The CRCI value in per replicate is calculated according to the following equation (Oksanen et al. 2006): CRCI = arcsin [(X – Y)/max (X, Y)], where X is the average biomass of the plants grown as a monoculture and Y is the average biomass of the plants grown in the mixture. A CRCI value >0 indicates that competition has a negative effect on the target plant, whereas a value <0 indicates that it has a positive effect.
Total carbon (C), nitrogen (N), phosphorus (P) and potassium (K) concentrations were measured in the leaves of both plant species (n = 8 per species and per treatment). Multiple mature leaves from one plant per treatment were collected from each replicate. For total C analysis, 20 mg of A. artemisiifolia or S. viridis leaves were used to measure the total C by using the potassium dichromate–concentrated sulfuric acid oxidation method (K2Cr2O7–H2SO4). Two grams of A. artemisiifolia or S. viridis leaves were used to analyze the N, P and K concentrations. N concentration was measured using the micro-Kjeldahl method (Nelson and Sommers 1973), whereas P and K concentrations were measured using inductively coupled plasma spectroscopy (Isaac and Johnson 1983).
Statistical analysis
In the field experiment, one-way analysis of variance (ANOVA) with a Duncan test was used to determine the differences in the extent of AMF root colonization (% colonized) on A. artemisiifolia and S. viridis roots when growing in a monoculture and as a mixture. Multiple comparisons (Duncan test) were used to determine the differences in AMF diversity (Shannon, Simpson and Evenness) index among the four different root treatments (A-mono, A-mix, S-mono and S-mix). The presence and absence of AMF phylotypes in each root sample were used to construct rarefaction curves to determine whether the sequencing depth was reasonable (with 95% confidence intervals) by using PAST software version 3.11 (Øyvind Hammer, Natural History Museum, University of Oslo, Norway). Non-metric multidimensional scaling (NMDS) was used to visualize compositional differences in the AMF communities among the root treatments by the Vegan package in R software version 2.5.2 (R Development Core Team, R Foundation for Statistical Computing, Vienna, Austria).
In the greenhouse experiment, the distribution of all variables was examined. The normality and homogeneity of the residuals were tested by visually checking the residual plot. A one-way ANOVA (Duncan test) was used to determine the effect of F. mosseae on the colonization rate (%), dry individual biomass, total carbon and N, P, and K concentrations of A. artemisiifolia and S. viridis roots when they were grown as a monoculture and as a mixture. Multiple comparisons (Duncan test) were used to determine the significant differences among different spore densities (C0, C1, C2 and C3) between these aforementioned variables and CRCI. A two-way ANOVA test was used to determine differences among the means for each of these variables (except for the colonization rate) and for the factors of AMF inoculum (C0, C1, C2 and C3) and interspecific competition. Pearson’s correlation analysis (two-sided test) was used to determine the relationship between the AMF colonization rate variables and plant growth indicators in the same treatment. All statistical analyses were conducted in SPSS 20.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
Field experiment: naturally occurring AMF communities in A. artemisiifolia and S. viridis roots
AMF colonization rate
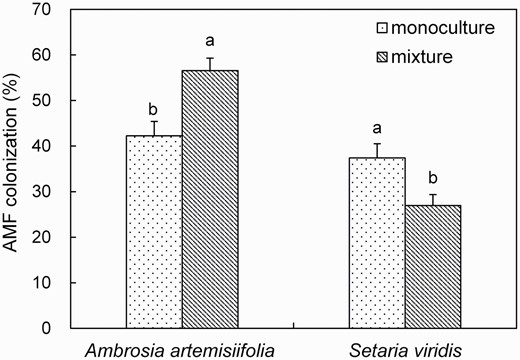
The colonization rate in the monoculture and mixture treatment of exotic species (A. artemisiifolia) was 42.23% and 56.58%, respectively. For S. viridis, the colonization rate in the monoculture and mixture treatment was 37.40% and 26.97%, respectively. Generally, the AMF colonization rate was higher in the exotic species (A. artemisiifolia) than in the native species (S. viridis) in both the monoculture (12.91%, F = 178.6, P < 0.001) and mixture treatment (109.79%, F = 236.5, P < 0.001). In addition, the colonization rate in the exotic species (A. artemisiifolia) in the mixture treatment significantly increased by 33.98% relative to its monoculture treatment (F = 121.1, P < 0.001). However, the colonization rate in the native species (S. viridis) in the mixture treatment significantly decreased by 27.89% relative to its monoculture treatment (F = 1705.1, P < 0.001, Fig. 1).
AMF colonization (%) in roots of exotic Ambrosia artemisiifolia or native Setaria viridis when grown in the monoculture of A. artemisiifolia or S. viridis, or in the mixture of A. artemisiifolia and S. viridis. Different letters in lower case indicate significant differences of the same plant species among different treatments at P <0.05. Error bars represent ± 1 SE of mean (n = 3).
AMF community structure
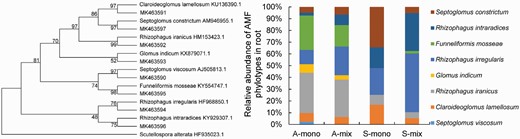
BLAST analyses showed that out of the 613 obtained sequences, 407 had a high degree of similarity to the AMF phylotypes (97%–100% identity), accounting for 66.4% of the total number. However, the other 206 sequences aligned with non-AMF phylotypes, mainly those belonging to the genera Isaria and Beauveria (data not shown) (Table 1). The rarefaction curves for the different root treatments reached saturation, indicating that the number of sequenced spores was reasonable (Supplementary Fig. S1). After performing phylogenetic analyses of the representative sequences using published AMF phylotypes, eight representative sequences of the clones were Septoglomus viscosum, Claroideoglomus lamellosum, Rhizophagus iranicus, Glomus indicum, Rhizophagus irregularis, F. mosseae, Rhizophagus intraradices and Septoglomus constrictum (GenBank accession numbers MK463950–MK463957) (Table 1; Fig. 2).
Number of clones detected for each AMF sequence type from each root treatment
| . | Treatments . | |||
|---|---|---|---|---|
| AMF phylotypes . | A-mono . | A-mix . | S-mono . | S-mix . |
| Septoglomus viscosum | 0.67 ± 0.33a | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a |
| Claroideoglomus lamellosum | 2.00 ± 0.00ab | 2.33 ± 0.33ab | 6.67 ± 0.88c | 1.67 ± 0.33a |
| Rhizophagus iranicus | 9.33 ± 0.88c | 11.67 ± 1.33d | 3.33 ± 0.67b | 1.67 ± 0.67a |
| Glomus indicum | 2.00 ± 0.58ab | 1.33 ± 0.33ab | 0.00 ± 0.00a | 0.00 ± 0.00a |
| Rhizophagus irregularis | 3.33 ± 0.33b | 9.00 ± 2.00cd | 9.00 ± 1.00d | 16.00 ± 1.00c |
| Funneliformis mosseae | 8.00 ± 0.58c | 6.67 ± 0.88c | 0.00 ± 0.00a | 0.67 ± 0.33a |
| Rhizophagus intraradices | 0.67 ± 0.33a | 3.33 ± 0.33b | 7.00 ± 0.58c | 10.33 ± 0.88b |
| Septoglomus constrictum | 1.33 ± 0.33a | 2.33 ± 0.88ab | 13.67 ± 0.33e | 1.67 ± 0.88a |
| Total number of AMF clones | 82 | 110 | 119 | 96 |
| Total number of non-AMF clones | 61 | 29 | 42 | 74 |
| Total number of clones | 143 | 139 | 161 | 170 |
| Total number of phylotypes | 8 | 7 | 5 | 6 |
| . | Treatments . | |||
|---|---|---|---|---|
| AMF phylotypes . | A-mono . | A-mix . | S-mono . | S-mix . |
| Septoglomus viscosum | 0.67 ± 0.33a | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a |
| Claroideoglomus lamellosum | 2.00 ± 0.00ab | 2.33 ± 0.33ab | 6.67 ± 0.88c | 1.67 ± 0.33a |
| Rhizophagus iranicus | 9.33 ± 0.88c | 11.67 ± 1.33d | 3.33 ± 0.67b | 1.67 ± 0.67a |
| Glomus indicum | 2.00 ± 0.58ab | 1.33 ± 0.33ab | 0.00 ± 0.00a | 0.00 ± 0.00a |
| Rhizophagus irregularis | 3.33 ± 0.33b | 9.00 ± 2.00cd | 9.00 ± 1.00d | 16.00 ± 1.00c |
| Funneliformis mosseae | 8.00 ± 0.58c | 6.67 ± 0.88c | 0.00 ± 0.00a | 0.67 ± 0.33a |
| Rhizophagus intraradices | 0.67 ± 0.33a | 3.33 ± 0.33b | 7.00 ± 0.58c | 10.33 ± 0.88b |
| Septoglomus constrictum | 1.33 ± 0.33a | 2.33 ± 0.88ab | 13.67 ± 0.33e | 1.67 ± 0.88a |
| Total number of AMF clones | 82 | 110 | 119 | 96 |
| Total number of non-AMF clones | 61 | 29 | 42 | 74 |
| Total number of clones | 143 | 139 | 161 | 170 |
| Total number of phylotypes | 8 | 7 | 5 | 6 |
Treatments: A-mono = Ambrosia artemisiifolia monoculture; A-mix = A. artemisiifolia in the mixture of A. artemisiifolia and Setaria viridis; S-mono = S. viridis monoculture; S-mix = S. viridis in the mixture of A. artemisiifolia and S. viridis. Data represent mean ± 1 SE (n = 3). Lower case letters indicate significant differences between each treatment (P < 0.05). The total number of AMF clones corresponds to the sequences which have high similarity to the AMF phylotypes, and the total number of non-AMF clones corresponds to the sequences which aligned with non-AMF phylotypes principally Isaria and Beauveria (data not shown).
Number of clones detected for each AMF sequence type from each root treatment
| . | Treatments . | |||
|---|---|---|---|---|
| AMF phylotypes . | A-mono . | A-mix . | S-mono . | S-mix . |
| Septoglomus viscosum | 0.67 ± 0.33a | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a |
| Claroideoglomus lamellosum | 2.00 ± 0.00ab | 2.33 ± 0.33ab | 6.67 ± 0.88c | 1.67 ± 0.33a |
| Rhizophagus iranicus | 9.33 ± 0.88c | 11.67 ± 1.33d | 3.33 ± 0.67b | 1.67 ± 0.67a |
| Glomus indicum | 2.00 ± 0.58ab | 1.33 ± 0.33ab | 0.00 ± 0.00a | 0.00 ± 0.00a |
| Rhizophagus irregularis | 3.33 ± 0.33b | 9.00 ± 2.00cd | 9.00 ± 1.00d | 16.00 ± 1.00c |
| Funneliformis mosseae | 8.00 ± 0.58c | 6.67 ± 0.88c | 0.00 ± 0.00a | 0.67 ± 0.33a |
| Rhizophagus intraradices | 0.67 ± 0.33a | 3.33 ± 0.33b | 7.00 ± 0.58c | 10.33 ± 0.88b |
| Septoglomus constrictum | 1.33 ± 0.33a | 2.33 ± 0.88ab | 13.67 ± 0.33e | 1.67 ± 0.88a |
| Total number of AMF clones | 82 | 110 | 119 | 96 |
| Total number of non-AMF clones | 61 | 29 | 42 | 74 |
| Total number of clones | 143 | 139 | 161 | 170 |
| Total number of phylotypes | 8 | 7 | 5 | 6 |
| . | Treatments . | |||
|---|---|---|---|---|
| AMF phylotypes . | A-mono . | A-mix . | S-mono . | S-mix . |
| Septoglomus viscosum | 0.67 ± 0.33a | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a |
| Claroideoglomus lamellosum | 2.00 ± 0.00ab | 2.33 ± 0.33ab | 6.67 ± 0.88c | 1.67 ± 0.33a |
| Rhizophagus iranicus | 9.33 ± 0.88c | 11.67 ± 1.33d | 3.33 ± 0.67b | 1.67 ± 0.67a |
| Glomus indicum | 2.00 ± 0.58ab | 1.33 ± 0.33ab | 0.00 ± 0.00a | 0.00 ± 0.00a |
| Rhizophagus irregularis | 3.33 ± 0.33b | 9.00 ± 2.00cd | 9.00 ± 1.00d | 16.00 ± 1.00c |
| Funneliformis mosseae | 8.00 ± 0.58c | 6.67 ± 0.88c | 0.00 ± 0.00a | 0.67 ± 0.33a |
| Rhizophagus intraradices | 0.67 ± 0.33a | 3.33 ± 0.33b | 7.00 ± 0.58c | 10.33 ± 0.88b |
| Septoglomus constrictum | 1.33 ± 0.33a | 2.33 ± 0.88ab | 13.67 ± 0.33e | 1.67 ± 0.88a |
| Total number of AMF clones | 82 | 110 | 119 | 96 |
| Total number of non-AMF clones | 61 | 29 | 42 | 74 |
| Total number of clones | 143 | 139 | 161 | 170 |
| Total number of phylotypes | 8 | 7 | 5 | 6 |
Treatments: A-mono = Ambrosia artemisiifolia monoculture; A-mix = A. artemisiifolia in the mixture of A. artemisiifolia and Setaria viridis; S-mono = S. viridis monoculture; S-mix = S. viridis in the mixture of A. artemisiifolia and S. viridis. Data represent mean ± 1 SE (n = 3). Lower case letters indicate significant differences between each treatment (P < 0.05). The total number of AMF clones corresponds to the sequences which have high similarity to the AMF phylotypes, and the total number of non-AMF clones corresponds to the sequences which aligned with non-AMF phylotypes principally Isaria and Beauveria (data not shown).
Neighbor-joining tree showing phylogeny of representative AMF DNA sequences isolated from root and reference sequences from GenBank. Fungal sequences from this study are identified by their GenBank sequences beginning with MK463590. Closely aligned sequences from GenBank are identified by the species name or accession number. Scutellospora alterata is used as the outgroup (left panel). Relative abundance of each AMF type detected from root sampled from different treatments (right panel). Treatments: A-mono =Ambrosia artemisiifolia monoculture; A-mix = A. artemisiifolia in the mixture of A. artemisiifolia and Setaria viridis; S-mono = S. viridis monoculture; S-mix = S. viridis in the mixture of A. artemisiifolia and S. viridis.
The composition of AMF species was sorted by NMDS, with the A. artemisiifolia and S. viridis treatment being clearly differentiated. NMDS ranking showed that the distribution of sampling points was similar to the community structure of the recorded AMF species (Supplementary Fig. S2). All treatments were clearly differentiated, with the replicates of each treatment being grouped together. One the one hand, the AMF phylotypes and their respective relative abundance differed among the four root treatments (Table 1; Fig. 2). Eight and seven AMF types were detected in A. artemisiifolia roots in the monoculture (A-mono) and in the mixture treatment (A-mix). Rhizophagus iranicus, F. mosseae, and R. irregularis were the dominant AMF in both A-mono and A-mix. The difference between A-mono and A-mix was that Sep. viscosum was not detected in the A-mix. In addition, R. irregularis and R. intraradices in the A-mix was higher than that in A-mono (FR. irregularis = 7.81, P < 0.05; FR. intraradices = 32, P = 0.005). Five and six AMF types were detected in the roots of S. viridis in the monoculture (S-mono) and in the mixture treatment (S-mix). Rhizophagus irregularis and R. intraradices were the dominant AMF in these two treatments, differing from the dominant AMF in A-mono. Funneliformis mosseae was detected in S-mix, whereas this species was not detected in S-mono. Multiple comparison among the four root treatments showed that F. mosseae in A-mono and A-mix was higher than that in S-mono and S-mix (F = 48.10, P < 0.0001, Fig. 2). On the other hand, the plots of the different treatments were correlated with different AMF species. Specifically, A-mono plots were closely correlated to F. mosseae and G. indicum, whereas A-mix plots were closely correlated to R. iranicus and F. mosseae. S-mono plots were correlated to C. lamellosum and Sep. constrictum, whereas S-mix plots were correlated to R. irregularis and R. intraradices. Thus, AMF communities clearly differed among the four root treatments. The differences in AMF communities between S-mono and S-mix indicated that the presence of A. artemisiifolia altered the composition of the AMF community in the roots of S. viridis.
Diversity of AMF community
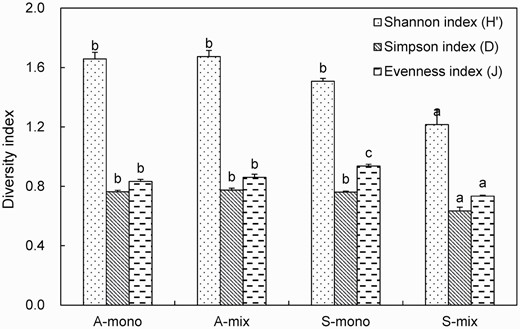
There were no differences in the Shannon, Simpson and Evenness indices between the A-mix and A-mono. However, the Shannon, Simpson and Evenness indices in S-mix were significantly lower than those in the other three root treatments (Fig. 3). These results further indicated that the presence of A. artemisiifolia altered the composition of the AMF community in the roots of S. viridis.
Shannon index (H′), Simpson index (D) and Evenness index (J) of AMF phylotypes in roots from different treatments. Treatments: A-mono = Ambrosia artemisiifolia monoculture; A-mix = A. artemisiifolia in the mixture of A. artemisiifolia and Setaria viridis; S-mono = S. viridis monoculture; S-mix = S. viridis in the mixture of A. artemisiifolia and S. viridis. Different lower case letters indicate significant differences of the same indicator among different treatments at P <0.05. Error bars represent ± 1 SE of mean (n = 3).
Greenhouse experiment: effect of AMF inoculum on interspecific competition between exotic and native plants
The field experiment showed that the A-mono and A-mix plots were closely correlated to F. mosseae. Moreover, F. mosseae colonized the roots of S. viridis in the presence of A. artemisiifolia but not in S-mono. Thus, we hypothesized that F. mosseae may contribute to the growth of A. artemisiifolia. To test this hypothesis, F. mosseae was inoculated at different spore densities to investigate how it influenced interspecific competition between A. artemisiifolia and S. viridis in a greenhouse pot experiment.
AMF colonization rate
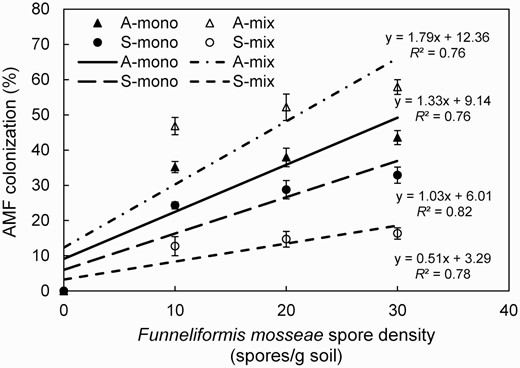
The dependency on dose increased in the F. mosseae inoculated roots of A. artemisiifolia when grown both in a monoculture (A-mono) and a mixture (A-mix) at different levels of AMF inoculation (Fig. 4). Specifically, AMF colonization at the three spore densities (C1, C2 and C3) was 35.16%, 37.91% and 43.56% in A-mono and 46.82%, 52.15% and 57.90% in A-mix, respectively. Furthermore, the AMF colonization rate in A-mix was higher than that in A-mono (C1: F = 401.1, C2: F = 242.2, C3: F = 611.1, all P < 0.001), suggesting the symbiosis between AMF and A. artemisiifolia was enhanced when A. artemisiifolia was grown with S. viridis.
AMF colonization (%) in roots of Ambrosia artemisiifolia or Setaria viridis when grown in the monoculture or mixture in soil inoculated with spores (C0: 0, C1: 10, C2: 20 or C3: 30 spores g−1 soil) of Funneliformis mosseae. Treatments: A-mono = A. artemisiifolia monoculture; A-mix = A. artemisiifolia in the mixture of A. artemisiifolia and S. viridis; S-mono = S. viridis monoculture; S-mix = S. viridis in the mixture of A. artemisiifolia and S. viridis. Error bars represent ±1 SE of mean (n = 8).
In native species (S. viridis), the root colonization rate followed a similar dose-dependent increasing trend to that of the exotic species (A. artemisiifolia) in both the monoculture (S-mono) and the mixture treatment (S-mix) with increasing spore density (Fig. 4). Specifically, AMF colonization was 24.29%, 28.75% and 32.89% in S-mono and 12.71%, 14.70% and 16.34% in S-mix at the three spore densities (C1, C2 and C3), respectively. However, the colonization rate in S-mix was lower than that in S-mono at all three spore densities (C1: F = 406.1, C2: F = 421.3, C3: F = 885.2, all P < 0.001). Interestingly, AMF colonization in A. artemisiifolia was greater than that in native S. viridis in both the monoculture (F = 8.54, P < 0.05) and mixture (F = 125.33, P < 0.001) treatment.
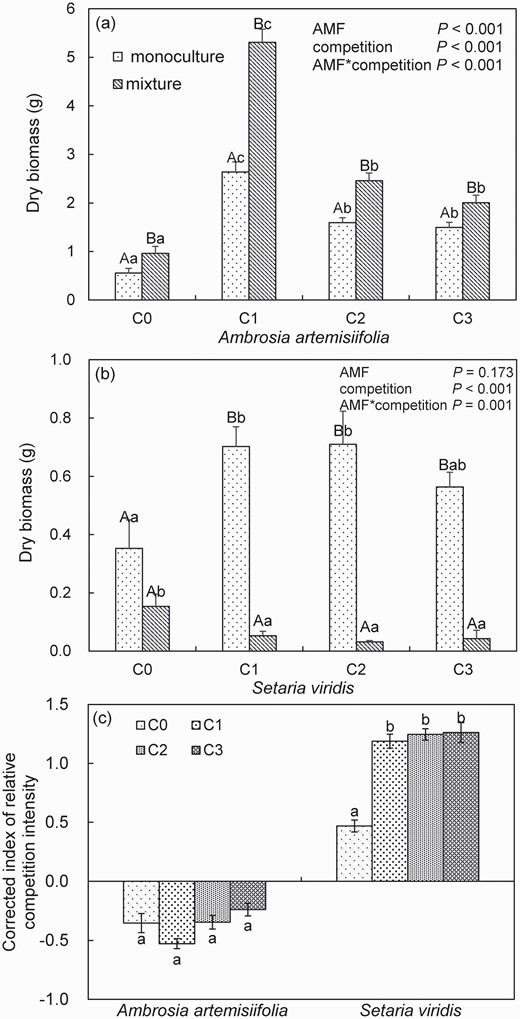
Plant biomass and CRCI
Compared with that in the monoculture, the dry biomass of A. artemisiifolia in the mixture increased by 72.90% in the control treatment (F = 5.54, P < 0.05). Compared with the biomass in the control treatment soil, the dry biomass of A. artemisiifolia in both the monoculture and mixture also significantly increased by 168.59%–373.75% and 108.35%–451.48% (all P < 0.001), respectively, when the soil was inoculated with F. mosseae spores at the three spore densities of C1, C2 and C3 (Fig. 5a). Moreover, inoculation levels did not influence the positive impact of interspecific competition on A. artemisiifolia (Fig. 5c).
Average of the individuals dry biomass (a, b) of Ambrosia artemisiifolia or Setaria viridis when grown in monoculture or mixture in soil inoculated with spores (C0: 0, C1: 10, C2: 20 or C3: 30 spores g−1 soil) of Funneliformis mosseae. Different lower case letters indicate differences between spore densities at P <0.05. Different uppercase letters indicate differences between mixture and monoculture at P <0.05. Error bars represent ± 1 SE of mean (n = 8). A two-way ANOVA is used to analyze the effect of AMF inoculums and interspecific competition at P <0.05. Error bars represent ± 1 SE of mean (n = 8). Effects of spores (C0: 0, C1: 10, C2: 20 or C3: 30 spores g−1 soil) of F. mosseae on the corrected index of relative competition intensity (CRCI) of A. artemisiifolia and S. viridis (c). CRCI value >0 indicates that competition has a negative effect, and CRCI value <0 indicates that competition has a positive effect on the target plant. Different letters within plant species indicate significant differences among various spore density treatments at P <0.05. Error bars represent ± 1 SE of mean (n = 8).
Compared with that in the control treatment, the dry biomass of S. viridis in monoculture increased by 59.84%–101.33% (all P < 0.05) across all levels of AMF inoculation. However, compared with that in the control treatment, the dry biomass of S. viridis in the mixture treatment significantly decreased by 65.62%–79.26% (all P < 0.05) in the AMF inoculation treatment. These results suggested that the growth of S. viridis was inhibited when S. viridis was grown with A. artemisiifolia, especially in AMF inoculation treatment (Fig. 5b). The corrected index for relative competition intensity further indicated that the presence of A. artemisiifolia had a negative effect on the growth of S. viridis, with this effect being higher in the inoculated treatment, regardless of the levels of inoculation (Fig. 5c).
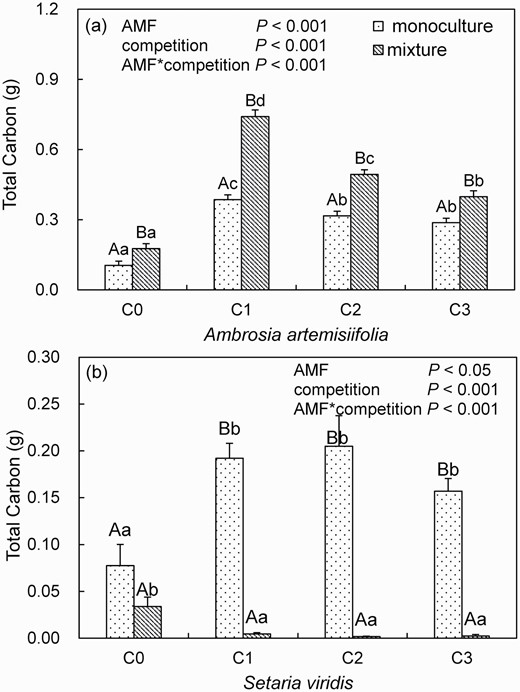
Total carbon content
The total carbon content of A. artemisiifolia increased when A. artemisiifolia grew with S. viridis. Its carbon content in the mixture increased by 67.99% relative to that in the monoculture in the control treatment (F = 6.93, P < 0.05, Fig. 6a). Funneliformis mosseae innoculation also increased its carbon content. When A. artemisiifolia grew in the inoculated soil, its total carbon content in the mixture significantly increased by 91.80%, 55.90% and 38.33% (C1: F = 99.55, C2: F = 40.49, C3: F = 12.42, all P < 0.01) at C1, C2 and C3, respectively, relative to that in the monoculture. Moreover, the total carbon content in A. artemisiifolia grown in monoculture or as a mixture in the F. mosseae inoculation treatment was higher than that in the control treatment.
Total carbon of Ambrosia artemisiifolia (a) or Setaria viridis (b) when grown in monoculture or mixture in soil inoculated with spores (C0: 0, C1: 10, C2: 20 or C3: 30 spores g−1 soil) of Funneliformis mosseae. Different lower case letters indicate differences between spore densities at P <0.05. Different uppercase letters indicate differences between mixture and monoculture at P <0.05. A two-way ANOVA is used to analyze the effect of AMF inoculums and interspecific competition at P <0.05. Error bars represent ± 1 SE of mean (n = 8).
Funneliformis mosseae inoculation increased the total carbon content of S. viridis when grown in monoculture. Total carbon of S. viridis in the monoculture increased by 102.38%–164.26% (all P < 0.01) in the inoculated soils compared with that in the control treatment soil. However, the total carbon content of S. viridis decreased in the mixture treatment both in the control treatment and in F. mosseae inoculation treatment. In the inoculated soil, the total carbon of S. viridis was significantly reduced by 97.65%, 99.12% and 98.48% when S. viridis grew with A. artemisiifolia, respectively (C1: F = 139.4, C2: F = 38.6, C3: F = 129.5, all P < 0.001), relative to that in the monoculture (Fig. 6b). The results suggested that the total carbon content in S. viridis decreased when S. viridis was grown with A. artemisiifolia, especially in the F. mosseae inoculation treatment.
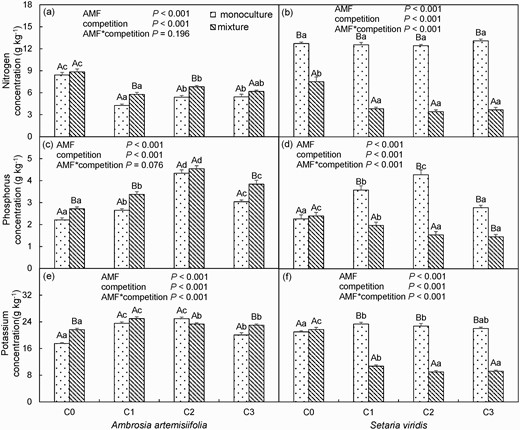
Nutrient absorption
The soil N concentration of A. artemisiifolia in the F. mosseae inoculation treatment was 35.48%–49.33% and 23.06%–34.79% (all P < 0.05) lower than that in the monoculture and mixture treatments, respectively (Fig. 7a). However, the P and K concentrations in A. artemisiifolia were higher in the F. mosseae inoculation treatment relative to that in the control treatment soil, regardless of whether they were grown in monoculture or mixture treatment (Fig. 7c and e).
Nitrogen (a, b), phosphorus (c, d) and potassium (e, f) concentrations of Ambrosia artemisiifolia or Setaria viridis when grown in monoculture or mixture in soil inoculated with spores (C0: 0, C1: 10, C2: 20 or C3: 30 spores g−1 soil) of Funneliformis mosseae. Different lower case letters indicate differences between spore densities at P <0.05. Different uppercase letters indicate differences between mixture and monoculture at P <0.05. Error bars represent ± 1 SE of mean (n = 8). A two-way ANOVA is used to analyze the effect of AMF inoculums and interspecific competition at P <0.05. Error bars represent ± 1 SE of mean (n = 8).
When the plants were grown in the mixture, compared with those in the control treatment, the concentrations of N, P and K in S. viridis in the F. mosseae inoculation treatment were reduced by 49.08%–54.41%, 17.98%–39.38% and 50.55%–58.47% (all P < 0.05), respectively (Fig. 7b, d and f). However, when grown in monoculture, compared with those in the control treatment, the foliar concentrations of both P and K in S. viridis were increased by 10 or 20 spore g−1F. mosseae inoculations (Fig. 7d and f). Thus, when the two species were grown together, AMF benefited the invasive species A. artemisiifolia but disadvantaged S. viridis in terms of P and K accumulation.
Correlations between root colonization rate and plant growth indicators
When A. artemisiifolia was grown in the monoculture and mixture treatment with S. viridis, the colonization rate in F. mosseae had a significantly positive correlation with dry biomass (rA-mono = 0.615, rA-mix = 0.470, both P < 0.01), total carbon (rA-mono = 0.770, rA-mix = 0.650, both P < 0.01), P (rA-mono = 0.577, rA-mix = 0.719, both P < 0.01) and K (rA-mono = 0.627, rA-mix = 0.486, both P < 0.01) concentrations, but a negative correlation with N concentration (rA-mono = −0.790, rA-mix = −0.781, both P < 0.01) (Supplementary Table S1). In the mixture treatment with A. artemisiifolia, the F. mosseae colonization rate in native species (S. viridis) had a significantly negative correlation with dry biomass (rS-mix = −0.575, P < 0.01), total carbon (rS-mix = −0.698, P < 0.01), N (rS-mix = −0.829, P < 0.01), P (rS-mix = −0.669, P < 0.01) and K (rS-mix = −0.959, P < 0.01) concentrations.
Discussion
In this study, we carried out a long-term experiment in which we compared AMF composition and diversity in the roots of A. artemisiifolia and S. viridis under field conditions. We then tested the effects of AMF on the growth of the exotic species A. artemisiifolia and its native competitor S. viridis using a greenhouse experiment. The results showed that AMF colonization in exotic species (A. artemisiifolia) was greater than that in native species (S. viridis). Meanwhile, the AMF community of the S. viridis roots when S. viridis was grown in the mixture with A. artemisiifolia was different from that when S. viridis was grown in the monoculture. Funneliformis mosseae, one of the dominant AMF in the root of A. artemisiifolia, facilitated the growth of the exotic A. artemisiifolia and reduced the competitiveness of the native S. viridis.
The strength of this study was the long-term continuous maintenance of a field plot with the investigated plants growing in monoculture or as a mixture. Studies have shown that after 8 years, plant invasions can change the AMF diversity and alter the symbiotic relationship with AMF (Pringle et al. 2009; Vogelsang and Bever 2009). AMF phylotypes and their relative abundance in A. artemisiifolia differed to those in S. viridis. Interestingly, the presence of A. artemisiifolia altered the mutualisms between native plants and AMF when plants were grown in mixture treatment. Specifically, F. mosseae was often found in the roots of A. artemisiifolia when it grew in monoculture or as a mixture with S. viridis. However, it was not detected in the roots of S. viridis grown in monoculture. Yet, F. mosseae colonized the roots of S. viridis when A. artemisiifolia grew alongside S. viridis. Thus, A. artemisiifolia altered the AMF community of native species and exploited specific beneficial interactions with mutualists to enhance their competitiveness. Several previous studies have also shown that exotic species can exploit different mycorrhizae from native species (Day et al. 2015; Moeller et al. 2015; Sun et al. 2013; Zhang et al. 2017), or can disrupt the mutualisms between AMF and native species, which benefit its competitive growth (Meinhardt and Gehring 2012). However, the manner in which this phenomenon influences the compartmentalization of AMF diversity among plant species is yet to be determined.
Previous studies have shown that trait-based partner selection may be a strong force in maintaining plant–AMF symbioses (Klironomos 2002, 2003; López-García et al. 2017; Menzel et al. 2018). Plants with different traits may have preferences for different AMF (Chagnon et al. 2015; van Kleunen et al. 2010). Differences in AMF associations between A. artemisiifolia and S. viridis may, therefore, be attributed to trait differences, rather than the plant species status as native or exotic. Asteraceae plants have a different AMF community composition and higher taxonomic diversity than that of Fabaceae plants because of differences in their plant traits (López-García et al. 2014; Scheublin et al. 2004). Ambrosia artemisiifolia is a C4 dicotyledonous forb (Asteraceae) species, whereas native S. viridis is a C4 grass (Poaceae) species, and the two species therefore likely differ in their functional traits (Zhang et al. 2018). Several studies have demonstrated that plant functional traits (such as plant morphology, plant phenology and root exudates) shape the fungal diversity in their roots (Alguacil et al. 2019; Chagnon and Bradley 2013; Hugoni et al. 2018; López-García et al. 2017; Öpik et al. 2009, 2013; Torrecillas et al. 2012; Vandenkoornhuyse et al. 2003). We have used the same consistent field conditions here as in our previous studies to obtain a long interaction time to determine the effect of exotic Fl. bidentis (L.) Kuntze. and native competitor S. viridis on the AMF communities in their roots. Both of Fl. bidentis and A. artemisiifolia belong to the family Asteraceae. The dominant AMF in the roots of Fl. bidentis is Rhizoglomus intraradices, which differs from that of A. artemisiifolia, even though they grow under the same conditions (Zhang et al. 2017). Thus, different exotic species may take advantage of specific AMF to facilitate their competitive growth.
The competitive growth of plants is closely related to their ability to fix carbon and absorb nutrients (Mantoani et al. 2020; Yu and He 2021). On the one hand, interspecific competition increases the total C content of A. artemisiifolia in the absence of F. mosseae. Funneliformis mosseae inoculation further promotes the ability of invasive A. artemisiifolia to fix C, especially when A. artemisiifolia competes with S. viridis. For S. viridis, interspecific competition decreases the total C content of the native species. In addition, compared with that in the control treatment, there is a significant increase in total C content in the monoculture of S. viridis; however, a significant decrease in total C content as a mixture occurs in the inoculated treatment, suggesting that F. mosseae inoculation enhances the inhibitory effect of interspecific competition on carbon sequestration of S. viridis when it grows with A. artemisiifolia. AMF are obligate biotrophs that receive their C supply from their host plant (Soudzilovskaia et al. 2015; Thirkell et al. 2020). The development of AMF extraradical hyphae and mycorrhizal colonization depends on C supply from their host plant (Bago et al. 2000; Cameron et al. 2006; Douds et al. 2000). In the current study, the increased total C content of A. artemisiifolia compared with that of S. viridis makes it easier for it to form mycorrhizal symbiosis with AMF, because it might allocate more C to AMF communities than S. viridis. Consequently, the AMF colonization rate in the invasive A. artemisiifolia is greater than that in the native species. In the greenhouse experiment, compared with native S. viridis, the greater ability of F. mosseae to colonize the roots of A. artemisiifolia might be attributed to the differences in C-allocation and nutrients absorbed by the AMF communities (Hart and Reader 2002; Lekberg et al. 2013). On the other hand, in response to inoculation with F. mosseae spores, there was a significant increase in A. artemisiifolia foliar P and K concentrations and a concomitant decrease in S. viridis foliar N, P and K concentrations when both plant species were in interspecific competition compared with intraspecific competition. Thus, AMF preferentially provide more nutrients to A. artemisiifolia, enhancing its competitive advantage. Specifically, F. mosseae provides A. artemisiifolia with a competitive advantage over S. viridis via P and K uptake.
Our results suggest that an exotic plant species has a stronger competitive ability than a native species, which may be related to differences in their AMF colonization rate. AMF influence the competitive ability of exotic and native species by changing their respective abilities to acquire soil nutrient resources. A significant increase in leaf P and K concentrations in A. artemisiifolia and a concomitant decrease in leaf total C, N, P and K concentrations in S. viridis in response to inoculation with F. mosseae spores are closely related to the AMF colonization rates when the plants grown in a mixture treatment. Thus, the AMF colonization in plant roots might be a good indicator of plant growth (Bunn et al. 2015; Cortois et al. 2016; Zhang et al. 2017). Our findings imply that AMF help A. artemisiifolia outcompete native S. viridis through F. mosseae, providing more resources to A. artemisiifolia compared with S. viridis because of its higher colonization in A. artemisiifolia (Fumanal et al. 2006; Zhang et al. 2018).
Supplementary Material
Supplementary material is available at Journal of Plant Ecology online.
Figure S1: Rarefaction curves for the NS31/AMmix sequences obtained for various root treatments.
Figure S2: Non-metric multidimensional scaling (NMDS) ordination comparing AMF phylotypes sampled among the root treatments.
Table S1: Correlation of plant growth indicators with root colonization rate.
Funding
This research was funded by the National Natural Science Foundation of China (grant no. 31972343 and 31372000), Hebei National Natural Science Foundation (C2019201059) and College of Life Science, Institute of Life Science and Green Development, Hebei University.
Acknowledgement
We thank Professor Jinli Zhao for providing the inoculum of F. mosseae.
Conflict of interest statement. The authors declare that they have no conflict of interest.
References
Author notes
These authors contributed equally to this work.