-
PDF
- Split View
-
Views
-
Cite
Cite
Sudip Gaire, Simona Principato, Coby Schal, Zachary C DeVries, Histamine Excretion by the Common Bed Bug (Hemiptera: Cimicidae), Journal of Medical Entomology, Volume 59, Issue 6, November 2022, Pages 1898–1904, https://doi.org/10.1093/jme/tjac131
Close - Share Icon Share
Abstract
The common bed bug (Cimex lectularius L.) is a hematophagous pest species that lives in close proximity to humans. Following a blood meal, bed bugs deposit fecal material indoors. The feces contain a variety of compounds, including histamine, which serves as a component of their aggregation pheromone. Histamine is a pivotal mammalian immune modulator, and recently it was shown to be present in high concentrations in household dust from homes infested with bed bugs. To better understand the dynamics of histamine excretion, we analyzed bed bug fecal material from different life stages and populations, along with fecal material collected at different post-feeding times and from bed bugs fed on different diets. Our analysis showed significant variation in histamine excretion among life stages, with mated females excreting the most histamine and first instar nymphs excreting the least histamine. However, when histamine excretion was normalized for blood consumption, males were found to excrete more histamine than the other life stages. There was no difference in histamine excretion among laboratory and recently home-collected bed bug populations. Further, we found histamine excretion continued for at least 14 d post-feeding, with the highest amount of histamine excreted 3–4 d after a bloodmeal. Overall, this work demonstrates that bed bugs excrete histamine across all feeding life stages, populations, and at various times after feeding, and that histamine excretion is directly related to blood feeding. These results will be used to better understand the health risks associated with histamine excretion and potential mitigation strategies of environmental histamine.
The common bed bug (Cimex lectularius L.) remains one of the most challenging indoor pests to control due to high levels of insecticide resistance and a cryptic life cycle (Potter 2011, Romero 2018). Worse, since their resurgence in the early 2000s, bed bugs have become one of the most common urban pests found indoors, particularly in disadvantaged communities (Wang et al. 2016, 2018). This is concerning, when we consider the effects bed bugs have on human health.
Bed bugs are formally considered pests of significant public health importance (CDC 2010). This is largely due to their propensity to feed on human blood and the resulting reactions to their bites (Goddard and Deshazo 2009, Pritchard and Hwang 2009, Doggett et al. 2012). People often develop rashes and other allergic responses following a bed bug bite, largely in reaction to bed bug salivary proteins (Valenzuela et al. 1996a,b; Price et al. 2012). Bed bugs have also been found to cause severe psychological effects, such as nightmares, insomnia, and anxiety, which may persist long after bed bugs are eradicated (Potter et al. 2010, Goddard and de Shazo 2012, Susser et al. 2012). Despite these effects, bed bugs have not been shown to naturally transmit any pathogens. Several studies have shown bed bugs are capable of transferring the causative agents of Chagas disease (Trypanosoma cruzi Chagas) and trench fever (Bartonella quintana Schmincke) in the laboratory, but transmission outside of the laboratory has not been documented (Leulmi et al. 2015, Salazar et al. 2015).
More recent public health concerns regarding bed bugs have arisen out of their ability to modify and/or introduce biocontaminants indoors. Specifically, Kakumanu et al. (2020) found that bed bugs were capable of influencing the indoor microbiome, causing it to differ significantly in composition from that of homes without bed bugs. Interestingly, this shift was found to be reversible with bed bug eradication. Prior to this, DeVries et al. (2018) found that settled dust in homes infested with bed bugs had significantly higher levels of histamine (average of 0.54 mg/g of dust) than in uninfested homes. However, unlike the microbiome, histamine persisted within house dust after bed bugs were eradicated, suggesting high stability indoors, and raising serious concerns about the impacts of exogenous histamine exposure on human health (Hargreave et al. 1981, van der Valk et al. 2015).
Histamine is found in bed bug feces and serves as a component of their aggregation pheromone, causing bed bug arrestment (Gries et al. 2015). Histamine is partially synthesized from histidine acquired from bloodmeals, but this only constitutes a fraction of the histamine they produce, suggesting histamine is primarily synthesized de novo (Gries et al. 2018). Outside of bed bugs, histamine is a biogenic amine that is synthesized in the human body and serves numerous biological functions such as neurotransmission, immunomodulation, and muscle cell contraction (Maintz and Novak 2007). In regards to immunomodulation, excess endogenous histamine release can lead to high blood pressure, headaches, vomiting, and respiratory distress (Maintz and Novak 2007, Thangam et al. 2018). Histamine is also considered a contaminant in food (fish) and alcoholic beverage (beer and wine) (Shalaby 1996). Cutaneous histamine exposure can result in dermatitis, whereas respiratory exposure can reduce forced expiratory volume and increase nasal mucosa activity, mostly in atopic patients (Curry 1946, Cockcroft et al. 1977, Heyer et al. 1989, Rudblad et al. 2002, Ständer and Steinhoff 2002). Despite histamine serving as a positive control in many allergen assessments, the risks of exogenous histamine exposure are largely unknown (Siegel et al. 1991, 1992; Kullman et al. 1998). However, clinical tests on dermal, nasal, and respiratory responses suggest that the presence of histamine indoors may pose a significant risk to human health (Hargreave et al. 1981, van der Valk et al. 2015).
Bed bugs clearly contribute histamine to the indoor environment (Gries et al. 2015, DeVries et al. 2018), but it is unclear what life stages are responsible, how excretion varies among populations, and how much histamine bed bugs excrete over time. It is also unclear what role ingesting human blood plays in histamine production. To better understand histamine excretion, we collected bed bug fecal material from different life stages, populations, times after feeding, and from bed bugs fed different diets and analyzed it for histamine content. The results are discussed in relation to potential indoor exposure risks.
Materials and Methods
Bed Bug Populations, Rearing, and Feeding
A laboratory-maintained colony of bed bugs (Harold Harlan [HH]) was used for all experiments. This population was originally collected in 1973 from Ft. Dix, NJ, USA. In addition, bed bugs collected more recently from two populations, LA-1 (collected for Los Angeles, CA, in 2006) and COL-3 (collected from Columbus, OH in 2019), were also used for selected experiments.
Bed bugs were reared under standard laboratory condition (25°C, 50% RH, and a photoperiod of 12:12 (L:D) h). They were maintained in plastic containers (size: 168 ml; Consolidated Plastics, Stow, OH) with folded cardstock paper serving as harborage material. Bed bugs were fed on human blood containing the anticoagulant citrate phosphate dextrose (CPD) solution (Kentucky Blood Center, Lexington, KY) using an artificial feeding system (Montes et al. 2002, Gaire et al. 2020). Briefly, blood was fed through custom-made water-jacketed glass feeders. Those feeders had a thin membrane (plant budding tape; A.M. Leonard, Piqua, OH) stretched over the bottom that contained the blood. Heated water (37°C) was continuously circulated through the outer chamber layer of glass to maintain blood temperature while feeding in the inner layer.
Histamine Excretion in Various Life Stages
Different bed bug (HH) life stages (males, mated females, unmated females, third instar nymphs, and first instar nymphs) were evaluated for their ability to produce histamine. Prior to feeding, all bed bugs were starved for 7 d, then placed into 4.5 ml vials (Thermo Fisher Scientific, Waltham, MA) with paper harborage that touched a mesh lid at the top of the vial (to allow for feeding). Adult bed bugs were fed individually, while nymphs were fed in groups (third instars in a group of 5; and first instar in a group of 20) to ensure accurate weights. After placing bed bugs into vials, the vials were placed under the artificial feeders, and bed bugs were allowed to feed until repletion (those bed bugs that partially fed were not used in this study). Bed bugs were weighed before and immediately after feeding to determine the amount of blood consumed. Weights were recorded by subtracting the weight of the vial from the weight of the vial and the bed bug(s). After feeding, bed bugs were left in the vials for 7 d, after which they were reweighed by transferring to new vial to determine the amount of body mass lost after feeding. The seven-day-old vials with fecal deposits were capped and stored at −20°C until analysis. In total, ten replications were done for each life stage. Control vials (with harborage but no bugs) were also placed adjacent to vials with bed bugs and analyzed for histamine.
Histamine Excretion in Different Populations
Two home-collected bed bug populations (Col-3 and LA-1) and one laboratory-reared population (HH) were used to determine if there were differences among bed bug populations in histamine excretion. The methods, including controls, followed those used to evaluate histamine excretion and various weights in different life stages of HH bed bugs (as described above), but only adult males were evaluated for each population. In total, ten replications were done for each population.
Histamine Excretion over Time
Adult male bed bugs (HH) were used to evaluate the time-course of histamine excretion after a bloodmeal. Prior to feeding, bed bugs were starved for 7 d, then placed individually into 4.5 ml vials with paper harborage that touched a mesh lid at the top of the vial, as described above. The vials were placed under the artificial feeders and bed bugs allowed to feed until repletion. Bed bugs were weighed before and immediately after feeding to determine the amount of blood consumed as described above “Histamine Excretion in Various Life Stages” section. After feeding, bed bugs were transferred to new (clean) vials every day for 14 d. Only samples from days 1–7, 10, and 14 were analyzed and bed bug weights were also taken for these days. The vials with fecal deposits were stored at −20°C until histamine analysis. In total, ten replications were done. For each replication, histamine was quantified at nine-time points (days 1–7, 10, and 14). The control samples that lacked bed bugs but contained similar harborage were collected similarly and placed into the freezer on day 14.
Effects of Feeding on Histamine Excretion
Bed bugs (individual adult male HH) were fed different solutions to determine the role of feeding and diet (blood) in histamine excretion. Prior to feeding, all bed bugs were starved for 14 d. A 14-day starvation period was used to minimize the amount of blood in their gut from the previous feeding and ensure histamine excretion was at a minimal level (based on the Histamine Excretion Over Time work [previous section]). After starvation, bed bugs were placed into 4.5 ml vials with paper harborage, as described above, and fed either human blood or 1X phosphate buffer saline (PBS, 7.4 pH). A third treatment group was not fed, but still evaluated for histamine excretion. Bed bugs were weighed before and immediately after feeding to determine the amount of blood or PBS consumed as described above in the “Histamine Excretion in Various Life Stages” section. After feeding, bed bugs were left in the vials for 7 d, after which they were reweighed to determine the body mass lost after feeding (e.g., the amount of blood or PBS that was processed). The vials containing fecal deposited harborages were capped and stored at −20°C until analysis. In addition, to determine if histamine may come directly from the blood, fresh human blood (2 mg) and PBS (2 mg) were placed into 4.5 ml vials and analyzed for histamine. This weight (2 mg) was approximately the bloodmeal size consumed by an adult male bed bug. In total, ten replications were done for each treatment.
Extraction, Derivatization, and Analysis of Histamine
Histamine was extracted, derivatized, and analyzed according to DeVries et al. (2018) with some modifications. Samples were removed from the freezer and allowed to reach room temperature. Water (1 ml, HPLC grade) was added to each sample, followed by 10 µg of mass labeled histamine (histamine-α,α,β,β-d4; CDN Isotopes, Quebec, Canada) as an internal standard (in 0.1 M HCL; Macron Fine Chemicals, Radnor, PA). Samples were then extracted by vortexing for 30 s, shaking on a rocker for 10 min, then centrifuging at 400 g for 5 min, with supernatants transferred to new vials. Next, 1 ml toluene (VWR Chemicals, Radnor, PA), 2 ml alkaline buffer (pH 12; Honeywell International Inc, Charlotte, NC), and 100 µL isobutyl chloroformate (Alfa Aesar, Haverhill, MA) were added to the supernatant and shaken on a rocker for 45 min. The top organic layer was transferred to a 1.5 ml glass vial, evaporated to dryness under N2, resuspended in 1 ml of toluene, and stored at −20°C until GC-MS analysis.
Histamine analysis was carried out using an Agilent Technologies (Santa Clara, CA) 8860 GC coupled to a 5977B MS operating in electron ionization mode. The GC was fitted with a 30 m × 0.25 mm × 0.25 µm HP-5MS UI column (Agilent Technologies). The temperature program was: 100 to 300°C at the rate of 30°C/min and then held for 5 min. The internal standard method of quantification was used with a 10-point calibration curve ranging from 0.1 to 100 µg/ml, and all samples were within the range of standards. Both internal standard (m/z 197) and histamine (m/z 194) ions were selected for quantifications, and these compounds appeared at approximately the same retention time (6.32 min).
Statistical Analysis
Histamine excretion quantity, normalized concentrations, and blood consumptions for life stages, populations, and starvation–feeding experiment were analyzed using one-way ANOVA followed by Tukey’s HSD test at the significance level of α < 0.05. The only exception was using a two-sample t-test for meal consumption in the starvation–feeding experiment to compare blood versus PBS feeding. The time-course of histamine quantity and bed bugs weight over time was analyzed using repeated-measures ANOVA followed by LSD test. Regression analysis was performed to compare the histamine excretion and body mass lost in various life stages to the corresponding blood consumptions. All analyses were performed in SPSS Statistics 26 (IBM Corp., Armonk, NY).
Results
Histamine Excretion among Different Life Stages
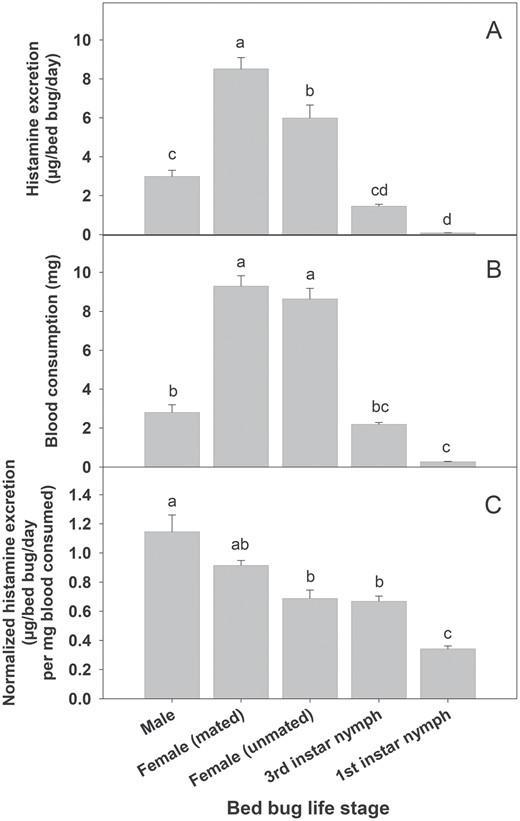
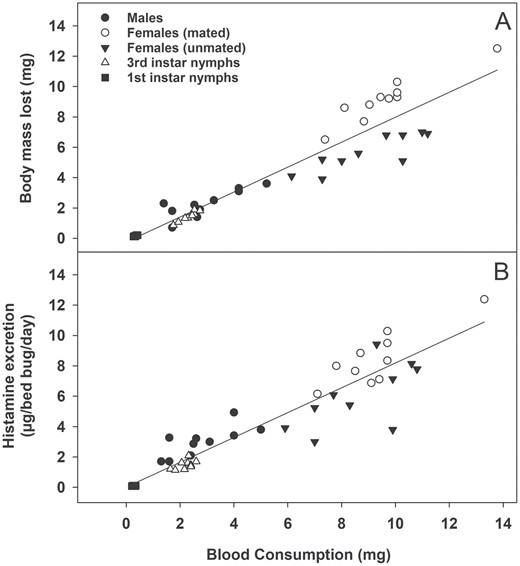
Total histamine excretion differed significantly among bed bug life stages (Fig. 1A; F4, 45 = 65.81, P < 0.001). Mated females excreted the most histamine, followed by unmated females, males, third instar nymphs, and finally first instar nymphs, which excreted the least histamine. Bloodmeal size also differed significantly among life stages (F4,45 = 115.05, P < 0.001), with those life stages that excreted more histamine also taking larger bloodmeals (Fig. 1B). When histamine excretion (µg histamine/bed bug/day) was normalized by blood consumption (i.e., µg histamine/bed bug/day/mg blood consumed), we found adult males excreted the most histamine (Fig. 1C; F4,45 = 23.47, P < 0.001), although the magnitude of differences among life stages was greatly reduced. Regression analysis confirmed a significant correlation between blood consumption and body mass lost (Fig. 2A; r2 = 0.904, F1,48 = 449.8, P < 0.001; body mass lost per bed bug [mg] = 0.011 (±0.255) + 0.818 (±0.043) * blood consumption per bed bug [mg], ± indicates SEM) as well as between blood consumption and histamine excretion (Fig. 2B; r2 = 0.885, F1,48 = 370.4, P < 0.001; bed bug histamine production [µg/bed bug/day] = −0.200 (±0.240) + 0.849 (±0.040) * blood consumption per bed bug [mg], ± indicates SEM).
Histamine excretion and blood consumptions among bed bugs life stages. (A) Total histamine excretion, (B) blood consumptions, and (C) blood consumption normalized histamine excretion. Different lowercase letters above bar graphs indicate significant differences between life stages (ANOVA followed by Tukey Test; P < 0.05).
Effects of blood consumption on (A) Body mass loss and (B) histamine excretion among bed bugs (all life stages combined). Blood consumption had a significant effect on both body mass loss and histamine excretion based on regression analysis (P < 0.001). See text for equations.
Histamine Excretion in Different Populations
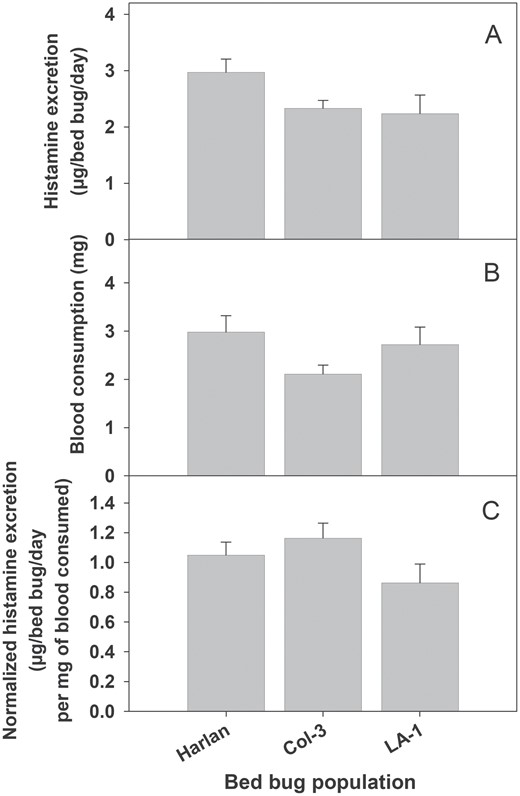
There were no differences in either total histamine excretion (F2,27 = 2.59, P = 0.093) nor blood consumption (F2,27 = 2.12, P = 0.140) among the laboratory (HH) and home-collected (Col-3 and LA-1) bed bug populations (Fig. 3). When histamine was normalized for blood consumption, we still did not find any differences in histamine excretion among the three bed bugs populations (F2,27 = 2.04, P = 0.149).
Histamine excretion by adult males from different population of bed bugs. (A) Histamine quantity excreted, (B) blood consumptions, and (C) blood consumption normalized histamine excretion. There was no significant difference in histamine excretion, blood consumption, or blood consumption normalized histamine excretion among various bed bug populations (ANOVA; P > 0.05).
Histamine Excretion over Time
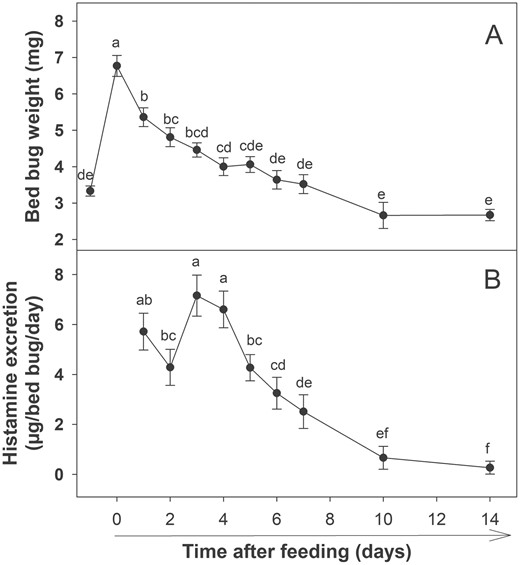
Time after feeding had a significant effect on both bed bugs weight (Fig. 4A) and histamine excretion (Fig. 4B). Bed bugs were found capable of excreting histamine for at least 14 d after a single bloodmeal (Fig. 4B). There was a significant difference in histamine excretion over time after a single bloodmeal (Fig. 4B; F8,72 = 19.63, P < 0.001). Although the overall trend in histamine excretion decreased over time, the highest quantity of histamine was excreted 3–4 d after a bloodmeal.
Histamine excretion by bed bugs over time after a single bloodmeal. (A) Bed bug weights after feeding. First and second data points represent average starved and fed weights, respectively. (B) Histamine excretion over time after a single bloodmeal. Different lower case letters indicate significant differences in body weights or histamine excretion between various time points (Repeated measures ANOVA followed by LSD test; P < 0.05).
Effects of Feeding on Histamine Excretion
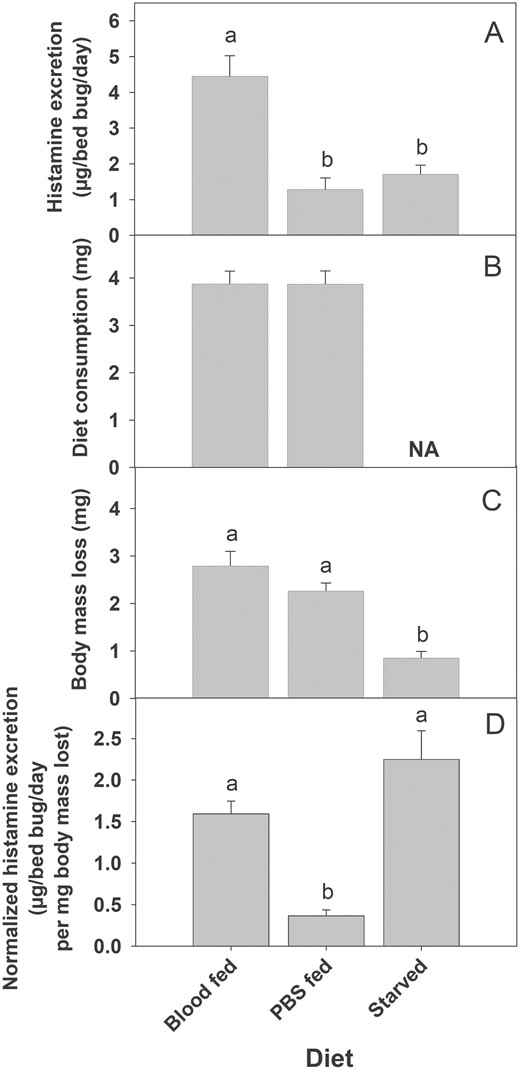
There was a significant difference in total histamine excretion among blood-fed, PBS-fed, and starved bed bugs (Fig. 5A, F2,27 = 18.08, P < 0.001), with bed bugs fed on blood excreting significantly more histamine, and no difference in total histamine excretion between bed bugs that were fed PBS or starved. However, there were no differences in meal size between bed bugs fed blood or PBS (Fig. 5B; t(18) = 0.026, P = 0.453). There was a significant difference in body mass loss among blood-fed, PBS-fed, and starved bed bugs (Fig. 5C, F2,27 = 23.19, P<0.001), with bed bugs that consumed either blood or PBS losing significantly more mass than those that were starved. When histamine excretion was normalized for body mass lost, there was no significant difference in histamine excretion between bed bugs fed on blood and those that were starved (Fig. 5D; F2,27 = 19.38, P = 0.102), although both were significantly higher than bed bugs fed on PBS (Fig. 5D; F2,27 = 19.38, P < 0.05). It should be noted that histamine was normalized for body mass lost rather than blood consumption due to the inclusion of a starved treatment group, which did not take a bloodmeal. However, the use of body mass loss rather than blood consumption is supported by the strong correlation between both variables (Fig. 1A). When analyzing blood and PBS, histamine was below the instrument’s method detection limit (MDL) (i.e., 0.16 µg/ml) for all samples.
Histamine excretion by bed bugs fed different diets. (A) Histamine quantity, (B) diet (blood or PBS) consumption, (C) body mass loss and (D) body-mass lost normalized histamine excretion. Different lower case letters above each bar (A, C, and D) indicate a significant difference between groups (ANOVA followed by Tukey Test; P < 0.05). There was no significant difference between blood and PBS consumptions (B) (t-test; P > 0.05).
Discussion
Bed bugs were found capable of excreting significant amounts of histamine. The total amount of histamine excreted differed significantly among life stages, with mated females producing the greatest amount of histamine. However, we also found the quantity of histamine excreted was directly correlated with blood consumption. For example, mated females excreted the most histamine, but they also took the largest bloodmeal. Similarly, first instar nymphs excreted the least histamine, but also consumed the smallest amount of blood. While this trend generally held true, we did find that both mated and unmated females consumed similar amounts of blood, but mated females excreted significantly more histamine than unmated females. This is likely due to metabolic differences between mated and unmated females, where mated females can immediately begin processing their bloodmeal and producing eggs while unmated females tend to retain their bloodmeal until mating has occurred (Usinger 1966, Saveer et al. 2021). This idea is further supported by the respiration rates of mated and unmated female bed bugs, with unmated females consuming almost 50% less O2 compared to mated females (DeVries et al. 2013) and showing a rapid decline in O2 consumption following feeding (DeVries et al. 2015), indicating a reduced metabolic rate which could result in reduced histamine biosynthesis. When total histamine excretion was normalized with blood consumption, we found that adult males excreted the most histamine relative to blood intake, whereas first instar nymphs excreted the least. However, it should be noted that the magnitude of differences among life stages was much smaller when normalized for blood consumption. It is also worth noting that when normalized for bloodmeal size, bed bug histamine excretion correlates very strongly with bed bug metabolism. Specifically, DeVries et al. (2013) found adult males had the highest metabolic rates and nymphal stages had the lowest metabolic rates, which matches histamine excretion when normalized with blood consumption. It should be noted that blood consumption and body mass loss were strongly correlated, and while we did not measure fecal excretion directly (due to logistical challenges with drying feces, accurately weighing fecal deposits, and analyzing for histamine), we speculate that increased bloodmeals lead to increased fecal deposits which likely drives histamine deposition indoors (Wilson and Miller 2022).
Histamine excretion was also compared among various bed bugs populations. Regardless of time in the laboratory, all three bed bug populations excreted similar amounts of histamine after feeding. Moreover, all populations took similar bloodmeal sizes, and thus when total histamine excreted was normalized for bloodmeal size, we also found no differences among populations. These results suggest that bed bug life history (time in the laboratory, insecticide resistance, etc.) does not impact histamine excretion and thus the ability of bed bugs to produce a component of their aggregation pheromone. However, this hypothesis should be further evaluated with a greater number and diversity of bed bug populations.
When evaluated over time, bed bugs were found capable of excreting histamine for at least two weeks following a single bloodmeal. While histamine excretion was high immediately following the bloodmeal, it declined steadily with time post-feeding (similar to decline in body weight, indicating digestion of blood relates to histamine production; Fig. 4A), except for days 1–4 after feeding. This pattern was very similar to the metabolic pattern observed for males by DeVries et al. (2015), where males were found to have a relatively stable metabolism for 3 d post-feeding, with declines observed starting on day 4. Given the metabolic data and histamine excretion data, we suspect that histamine production may be energetically costly and thus responsible for this correlation, but this has not been tested.
Given the importance of feeding to histamine excretion, we also evaluated whether the bloodmeal itself was responsible for driving histamine excretions. Bed bugs fed human blood excreted significantly more histamine than bed bugs fed a PBS solution, despite no significant difference in consumption of these two diets. Further, bed bugs fed the PBS solution did not show any differences in histamine excretion from those bed bugs that were not fed. These results indicate that blood consumption is the most critical factor driving histamine production, not just processing of the diets they consumed (e.g., histamine accumulation and deposition with feces regardless of diet). This also allows us to speculate that the qualitative nature of the meal may have a significant impact on histamine production, but this remains to be tested. In humans (Parsons and Ganellin 2006), locust (Elias and Evans 1983), and bacteria (Landete et al. 2008), histamine is produced through the decarboxylation of histidine using a histidine decarboxylase enzyme. Gries et al. (2018) explored this idea, finding that while bed bugs can convert histidine to histamine, histidine only comprised a small proportion of the histamine produced by bed bugs, suggesting that most of the histamine was produced de novo. Our experiment with blood and PBS diets confirmed that blood is needed for histamine production, but the role it plays as either an energy source, substrate for de novo biosynthesis, or source of histidine (or all three possibilities) remains unknown.
To accurately measure histamine excretion, we used only single bed bugs (or small groups in the case of nymphs), but this is not necessarily reflective of what would be encountered in an actual infestation. Wang et al. (2009) reported through visual inspection 87 out of 225 apartments in a high rise building that infestations ranged from 10 to 3,025 bed bugs per unit. The actual number of bed bugs in a home is extremely difficult to estimate because trap catches and visual inspections only reflect a percentage of the active population. That said, the number of bed bugs that can be found in a single home often number in the hundreds to thousands, especially in low-income housing where bed bug interventions are often performed poorly or lack altogether (Cooper and Wang 2018). In a hypothetical infestation with 1,000 bed bugs (500 adult males and 500 adult females), as much as 40 mg of histamine could be excreted in one week. Given that each bed bug will likely take a bloodmeal once a week, and histamine has been shown to be relatively stable in the indoor environment (DeVries et al. 2018), >2 g of histamine could accumulate by these bugs alone in just one year, not considering the natural growth of the population.
Histamine is reported as a highly stable molecule, and it degrades only 35% in 10 months at 30°C in food such as fish (Naila et al. 2012). Since bed bugs live close to humans (beds, sofas, and other resting areas), their excretion of histamine might pose a serious risk to humans due to likely direct contact when compared to other arthropod-produced allergens. However, the health effects related to indoor exposure to exogenous histamine remain unknown.
Bed bugs not only infest human homes, they also infest animal producing facility, specifically poultry houses (Szalanski 2018). In visual monitoring, it has been reported that there can be more than 100,000 bed bugs in a single poultry barn (~40,000 hens per barn) (Erasmus et al. 2019), with some barns exceeding one million at times (Gaire, personal observation). In such a scenario, histamine excretion and accumulation could be very high. This is concerning for poultry operations, since histamine has been shown to directly impact egg production when chickens consume a diet with as little as 0.25% histamine (Shifrine et al. 1963). In poultry houses, bed bugs live very close to chickens (e.g., cage frames, support wooden base etc.) (S.G., personal observation) and their feces containing histamine could contaminate diets. In the future, it will be important to investigate how bed bug produced histamine impacts egg production in poultry operations via contact or consumptions.
In conclusion, bed bugs possess a robust ability to excrete histamine across life stages and populations, and histamine can be deposited into the environment for at least 2 weeks following a single bloodmeal. Further, we confirmed that blood plays a critical role in histamine production. While bed bugs clearly contribute histamine to the indoor environment, there are other Cimex species and many indoor pests which could also contribute to indoor environmental histamine contamination; therefore, it is essential to evaluate if other pests can contribute histamine to the indoor environment. Given how much histamine bed bugs can excrete and its high stability indoors, it is critical that we investigate the impacts of exogenous histamine exposure on human and animal health.
Acknowledgments
We would like to thank Angela Sierras, Johnalyn Gordon, Henry Morgan, Kenneth Haynes, and Scott Bessin from the University of Kentucky, and Rick Santangelo from North Carolina State University for their assistance, inputs, and technical support on this project. This work was funded by the National Institutes of Health Director’s Early Independence Award (DP5-OD028155 to ZCD). CS was supported in part by the Blanton J. Whitmire Endowment, the Department of the Army U.S. Army Contracting Command (Aberdeen Proving Ground, Natick Contracting Division, Ft Detrick MD), and by the National Institute of Environmental Health Sciences of the National Institutes of Health under Award Number P30ES025128. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.