-
PDF
- Split View
-
Views
-
Cite
Cite
Jacob A Esselstyn, Anang S Achmadi, Heru Handika, Thomas C Giarla, Kevin C Rowe, A new climbing shrew from Sulawesi highlights the tangled taxonomy of an endemic radiation, Journal of Mammalogy, Volume 100, Issue 6, 19 December 2019, Pages 1713–1725, https://doi.org/10.1093/jmammal/gyz077
Close - Share Icon Share
Abstract
We describe a new species of Crocidura (Soricidae) from Sulawesi Island, Indonesia, documenting its novelty with both genetic and morphological characters. The new species is widespread on the island, with vouchered records from nine general localities distributed among five of the island’s areas of endemism. Morphologically, the new species is readily distinguished from all other described Sulawesi Crocidura by its intermediate body size, gray pelage, and long, hairy tail. The new species was mainly captured in pitfalls placed in the ground, but we also obtained evidence that it readily climbs trees and may be scansorial in its locomotor habits. Populations of the new species sampled from across the island are closely related, separated by < 0.02 uncorrected mitochondrial p-distances. The new species is one member of an endemic radiation of shrews on Sulawesi now known to contain six valid species and several undescribed species, all within the genus Crocidura. Resolution of species limits and phylogenetic relationships in this radiation is hindered by habitat loss at type localities, historical designation of new species using very small sample sizes, and a lack of genetic data from type specimens.
Kami mendeskripsikan spesies baru Crocidura (Soricidae) dari Pulau Sulawesi, Indonesia, sekaligus mendokumentasikan keunikan karakter secara genetik maupun morfologi dari spesies tersebut. Spesies baru ini tersebar luas di Pulau Sulawesi, diketahui berdasarkan spesimen yang berasal dari sembilan lokasi umum yang tersebar di lima kawasan endemik di pulau tersebut. Secara morfologi, spesies baru ini dapat dibedakan dari spesies Crocidura lainnya dari Sulawesi berdasarkan ukuran tubuh yang sedang, rambut tubuh berwarna abu-abu, dan ekor yang panjang dan berambut. Spesies baru ini sebagian besar diperoleh dari perangkap sumuran yang ditanam didalam tanah, selain itu kami juga mendapatkan bukti bahwa spesies ini mampu memanjat pohon dan kemungkinan memiliki perilaku sebagai pemanjat. Beberapa populasi spesies yang dikoleksi dari Sulawesi ini mempunyai kekerabatan yang dekat, hanya dipisahkan oleh jarak proporsi DNA mitokondria (tidak terkoreksi) sebesar < 0.02. Spesies baru ini merupakan salah satu anggota dari suatu kelompok radiasi endemik cecurut di Sulawesi yang sampai saat ini diketahui terdiri atas enam spesies yang valid, dan beberapa spesies yang belum dideskripsikan, semuanya termasuk didalam genus Crocidura. Kepastian dalam menetapkan batasan jarak antar spesies dan hubungan kekerabatan genetik dari radiasi kelompok cecurut di Sulawesi terkendala oleh kerusakan habitat pada lokasi spesimen tipe, sejarah penamaan spesies yang hanya berdasarkan sampel yang sedikit, dan keterbatasan data molekuler dari spesimen tipe.
White-toothed shrews in the genus Crocidura Wagler, 1832, a species-rich group distributed across Africa and Eurasia, represent one of the least resolved components of mammalian taxonomy. Currently, 198 species of Crocidura are recognized, far more than are known from any other mammalian genus (Myotis contains 134 species; Rattus contains 70 species—Burgin et al. 2018; American Society of Mammalogists 2018). While generic definitions are inherently arbitrary, the large number of species allocated to this genus, with an estimated crown age of only ~8 Ma (Butler 1998; Dubey et al. 2007), represents an implicit acknowledgment of the limited morphological variation that has evolved among these many species. Such low levels of morphological differentiation make it essential to combine genetic and phenotypic data to illuminate species limits and relationships. Several recent studies have employed these integrative approaches, and each has made progress in documenting diversity (e.g., Esselstyn et al. 2014; Ceríaco et al. 2015; Stanley et al. 2015; Demos et al. 2017). In some such studies (e.g., Demos et al. 2017), this work has been made more challenging by the small sample sizes routinely used in taxonomic descriptions in the early 20th century and the subsequent, widespread habitat loss that now prevents the collection of new topotypes.
Although most species of Crocidura are endemic to Africa, many are also found in island Southeast Asia (Ruedi 1995; Hutterer 2005a; Esselstyn et al. 2009; Demos et al. 2016). Most of these are restricted to areas west of Wallace’s Line. However, a small endemic radiation of Crocidura is known from Sulawesi Island, which lies just east of this stark biogeographic boundary. Six species (C. elongata, C. lea, C. levicula, C. musseri, C. nigripes, C. rhoditis) and one subspecies (C. nigripes lipara) have been described from the island (Miller and Hollister 1921; Ruedi 1995). All except C. nigripes are considered endemic to Sulawesi (Miller and Hollister 1921; Ruedi 1995; Fabre et al. 2018). On Sulawesi, C. elongata is known from sites spread across the island (Eldridge et al. 2018), but other species, last treated by Ruedi (1995), have not been reported so widely. Crocidura musseri is known only from the type locality on Mt. Rorekatimbo in central Sulawesi; C. rhoditis is reported from southwest, central, and northeast Sulawesi; C. nigripes is known from northern and central Sulawesi; C. lea is reported from northern and central Sulawesi; and C. levicula has been reported from central and southeast Sulawesi (Musser 1987; Miller and Hollister 1921; Ruedi 1995). However, these putative distributions are based on a relatively small number of specimens because none of the early small mammal inventories used trapping techniques that are effective for capturing shrews (i.e., pitfalls). As such, these species’ ranges are crude approximations at best. Phylogenetic estimates suggested that five Sulawesi species form a clade and hence are thought to have diversified in situ on the island, while C. nigripes appears to have arrived independently, and much later (Ruedi et al. 1998; Esselstyn et al. 2009). However, both Ruedi et al. (1998) and Esselstyn et al. (2009) used only one specimen per named species, and the latter authors were unable to assign a formal name to three species-level lineages, suggesting substantial uncertainty in the current taxonomy. A few authors have hinted at a need for taxonomic revision of Sulawesi’s Crocidura fauna by mentioning the presence of undescribed species (Musser 1982, 1987), lamenting the paucity of specimens (Ruedi 1995), or documenting extensive phylogeographic diversity within a single described species (Eldridge et al. 2018).
Since 2010, we have been collecting shrew specimens across Sulawesi and assessing both genetic and phenotypic traits to better understand the diversity, relationships, and ecology of these animals. Our studies have reinforced the notion that the taxonomy of Sulawesi’s shrew fauna—only the genus Crocidura is native to the island—does not adequately reflect its evolutionary diversity. During our surveys, we noted the presence of a phenotypically distinctive, easily recognizable species of shrew that is yet to be named, and we document its existence herein.
Materials and Methods
Sample collection
Since 2010, we have collected specimens of shrews from 11 mountains spread across Sulawesi. These samples are drawn from five of the island’s seven recognized areas of endemism (AoEs—Evans et al. 2003). We trapped in and around forest habitats in these areas using Victor rat traps and pitfalls. Most pitfall trapping employed large buckets (20- or 30-liter) with drift fence, but some of the effort relied on smaller buckets (3-liter) and used natural objects such as fallen trees as drift fence. We preserved specimens in formalin, often with the skull removed and cleaned, or as dried skins with cleaned skulls and skeletons. Tissue samples were frozen in liquid nitrogen, or fixed in ethanol or a noncommercial mix of RNAlater. These specimens are deposited at the Museum Zoologicum Bogoriense (MZB, Bogor), Field Museum of Natural History (FMNH, Chicago), Louisiana State University Museum of Natural Science (LSUMZ, Baton Rouge), University of California Museum of Vertebrate Zoology (MVZ, Berkeley), and Museums Victoria (NMV, Melbourne). All animal handling was conducted consistent with the guidelines of the American Society of Mammalogists (Sikes et al. 2016).
During our field surveys, we noted the occurrence of a distinctive species of shrew with an unusually hairy tail that did not fit the description of any known taxon from Sulawesi, or Southeast Asia more generally. While this new species has been obvious to us, some of the other shrews we collected have been extremely difficult to identify in relation to existing species names. In particular, the following situations make placing some specimens into formally named taxonomic units problematic: 1) we have not been able to confidently identify small shrews (< 5 g) that are potentially assignable to either C. lea or C. levicula; 2) multiple species resembling C. elongata are present on Sulawesi (Musser 1982; Ruedi and Vogel 1995; Eldridge et al. 2018; also see below); 3) a lack of genetic data from type specimens or type series for five of Sulawesi’s six species of Crocidura means we cannot explicitly and objectively link name bearing types with recently sampled populations; and 4) although we can often delimit all of the species at one locality (but not necessarily assign existing names to those units), complex patterns of genetic diversity and phenotypic conservation or convergence prevent us from linking evolutionarily distinct units between sites. Given these difficulties, we use colloquial names herein for some of the species in our comparative set and we focus our comparisons on the shrew fauna of a single mountain.
Taxon sampling and morphological evidence
We demonstrate the existence of a new species of Crocidura by distinguishing it morphologically from the holotypes (C. elongata, C. lea, C. levicula, C. nigripes, C. n. lipara, and C. rhoditis) or from specimens we collected near the type locality (C. musseri) of all named species of shrew from Sulawesi. Furthermore, we use genetic data to 1) show that the new species is distinct from Crocidura species known from across Southeast Asia, and 2) to help define species limits in sympatric taxa on one Sulawesi mountain (Mt. Dako). For this latter use, we employed a process of reciprocal illumination between morphology and mitochondrial DNA sequences, and then tested the resulting hypothesis using nuclear DNA sequences. Results were consistent among data sets and we therefore compared the new species morphologically to each of these integratively defined, sympatric species sampled on Mt. Dako.
We captured two species of large, long-tailed shrews on Mt. Dako that resemble C. elongata, one of which has a paler pelage and feet than the other. However, we are unsure which, if either, is synonymous with true C. elongata (type locality: Temboan, North Sulawesi). When we refer to “C. elongata” below, we mean C. elongata sensu lato, including the holotype and both species from Mt. Dako. When we are referring to only one of the species from Mt. Dako, we use either “pale elongata” or “dark elongata,” as appropriate.
Similarly, two species of very small shrew (< 5 g), one paler than the other, are referred to below as “pale lea” and “dark lea.” We tentatively associate these specimens with C. lea because of their small body size, their proportionately similar tail length to the holotype of C. lea, their hind foot length of ~12 mm (Ruedi 1995), the small size of U2 relative to U3 (Ruedi 1995 found this to be a useful character), the relatively pale feet in both species and the C. lea holotype (as compared to the holotype of C. levicula), and because the type locality (Temboan) of C. lea is on the north peninsula. However, we note that tails of dark lea and pale lea are both more hirsute than in the holotype of C. lea, and similar in this condition to the type of C. levicula. Either or both species may belong to an undescribed species, or perhaps one represents C. levicula. However, we doubt the latter possibility primarily because the holotype of C. levicula has a shorter tail and darker feet, and the type locality is biogeographically distant from Mt. Dako (see Evans et al. 2003 and Giarla et al. 2018 for discussions of biogeographic distance on Sulawesi). When we refer to “C. lea” below, we mean the holotype and both species from Mt. Dako. Otherwise, we will specify when we are referring to pale lea, dark lea, or the holotype of C. lea.
We gathered standard external measurements (total length, tail length, hind foot length, ear length, and mass) from the field notes of collectors, specimen tags, and for C. musseri, we took the mean values from the original description of Ruedi (1995). We measured 12 craniodental dimensions from all seven Crocidura species we found on Mt. Dako, from all available skulls of the new species sampled from across Sulawesi, and from the holotypes of all known Sulawesi species except C. musseri. Cranial and dental measurements were taken by JAE using digital calipers accurate to 0.01 mm. These measurements comprise condylo-incisive length, braincase breadth, interorbital breadth, rostral length, post-palatal depth, rostral width, post-palatal length, condyle to glenoid length, length of upper tooth row (at crown), P4 to M3 crown length, labial M2 to M2 crown width, and palatal width. Measurements were taken as illustrated in figure 2 of Ruedi (1995). We follow the dental homology hypothesized by Hutterer (2005b) in regarding the upper three unicuspids of Crocidura as I2, I3, and C and the lower unicuspid as i2. Accordingly, the dental formula of Crocidura is 3 1 1 3 / 2 1 0 3 = 28.
To visualize morphometric variation and understand how the new species differs from other taxa and varies across the island, we made bivariate plots of standard external measurements, and we analyzed craniodental dimensions using principal components analysis (PCA). PCAs were conducted in R 3.5 (R Core Team 2018) using the correlation matrix on untransformed measurements. One PCA compared all species sampled from Mt. Dako and the holotypes of all named Sulawesi species except C. musseri. This PCA used 10 of the cranial dimensions (M2 to M2 and palatal width were excluded due to missing data). We conducted a second PCA comparing all skulls of the new species (i.e., multiple localities included) and using all 12 cranial dimensions.
Genetic evidence
We extracted whole genomic DNA using Qiagen DNEasy Blood & Tissue (Qiagen, Germantown, Maryland) or Promega Wizard (Promega Corp., Fitchburg, Wisconsin) kits, following the manufacturers’ recommended protocols. We used the polymerase chain reaction (PCR) to amplify targeted genetic regions. We targeted loci used in previous studies (Esselstyn et al. 2013; Demos et al. 2016) to facilitate the use of existing data and take advantage of previous efforts to optimize reaction conditions. Following the protocols of Esselstyn et al. (2009, 2013), we sequenced portions of one mitochondrial protein coding gene (cytochrome b: Cytb) and eight nuclear loci (seven exons: apolipoprotein B [Apob], brain-derived neurotrophic factor [Bdnf], breast cancer susceptibility 1 [Brca], growth hormone receptor exon 10 [Ghr], prostglandin E4 receptor [Ptger4], recombination activating protein 1 [Rag1], and von Willebrand factor exon 28 [vWf]; and one intron: mast cell growth factor [Mcgf]). We collected the mitochondrial sequence data from individuals of the new species from all sites where we know it occurs, and from representatives of all other Crocidura species that we collected on Mt. Dako (Fig. 1). We combined these new mitochondrial sequences with previously published sequences (one individual per species) selected from Demos et al. (2016). Nuclear genes were sequenced from multiple individuals of each of the seven Crocidura species from Mt. Dako. All sequences were aligned using Geneious 7.1.5 and examined by eye, with coding sequences checked for premature stop codons. Heterozygous sites in nuclear sequences were scored as ambiguities.
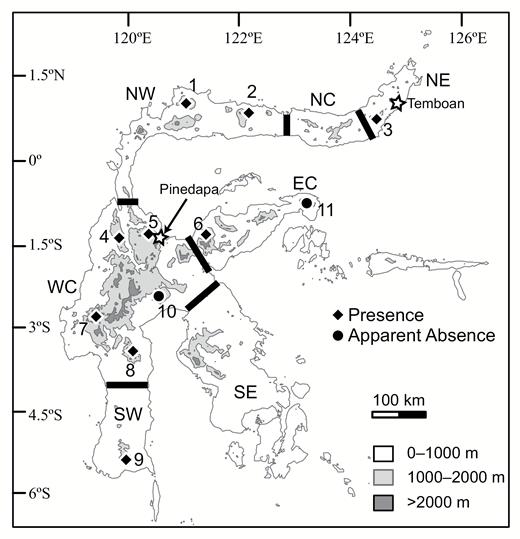
Map of Sulawesi, showing the distribution of Crocidura caudipilosa sp. nov. Sites with records of this new species are numbered: 1 Mt. Dako, 2 Mt. Buliohuto, 3 Mt. Ambang, 4 Mt. Torompupu, 5 Mt. Rorekatimbo, 6 Mt. Katopasa, 7 Mt. Gandang Dewata, 8 Mt. Latimojong, 9 Mt. Bawakaraeng, 10 Mt. Balease, and 11 Mt. Tompotika. Stars indicate the approximate locations of Temboan and Pinedapa, the type localities of previously described species. Black lines divide the island into its constituent areas of endemism, which are labeled NE (Northeast), NC (North-Central), NW (Northwest), WC (West-Central), EC (East-Central), SW (Southwest), and SE (Southeast).
We used PartitionFinder 2.1.1 (Lanfear et al. 2012, 2016) to simultaneously estimate the best model(s) of sequence evolution and the optimal partitioning scheme for the mitochondrial data. This alignment was divided by codon position (maximum of three partitions) for input to PartitionFinder, and we assessed the relative fit of models available in MrBayes (Ronquist et al. 2012) using the small sample size correction of Akaike’s information criterion (AICc), linked branch lengths, and the greedy search algorithm. We then implemented the best-fit models and partitioning scheme in MrBayes 3.2 to obtain an estimate of the mitochondrial gene tree topology and branch lengths. We used a single sequence from Crocidura monax (FMNH 173776; GenBank KP061993) from Stanley et al. (2015) as an outgroup because it is a distant relative of east Asian Crocidura. The analysis was run for 5 × 106 generations, with four Markov chain Monte Carlo runs, each with four chains (“Temperature” = 0.1), and samples were drawn every 2,000 generations. We then determined an appropriate burn-in by examining trace plots and sample sizes using Tracer 1.6 (http://tree.bio.ed.ac.uk/software/tracer/).
To estimate the species tree and best species delimitation scenario, we used BPP 3.4 (Yang and Rannala 2014; Yang 2015) to analyze nuclear DNA (eight loci, 45 individuals) from all seven Crocidura species collected on Mt. Dako. Individuals were assigned to the seven species we defined through reciprocal examination of the external phenotype and the mitochondrial gene tree topology. Replicated analyses were run with sampling every two generations, a pre-burn-in phase of 4,000 generations, and a posterior of 2 × 105 samples. Each analysis was initiated using the mitochondrial gene tree topology as the starting tree. We completed two independent analyses for each of three prior combinations on the mutation-rate-scaled effective population size (θ) and root divergence time (τ). BPP 3.4 uses an inverse gamma distribution on these priors. We ran the analyses using the default priors (θ: α = 3, β = 0.004; τ: α = 3, β = 0.002) and two modifications that doubled the mean and made both priors more diffuse by modifying the shape parameter (θ: α = 2, β = 0.004; τ: α = 2, β = 0.002) or increased the mean root age by a factor of 10 by modifying the scale parameter (θ: α = 3, β = 0.004; τ: α = 3, β = 0.02). Initially, we simultaneously estimated the species tree and species limits. Because all analyses delimited all seven morpho-mitochondrial species with a posterior probability of 1.0 and produced the same best topology, we then fixed the species limits and returned all priors to their defaults to estimate only the species tree.
Results
Fieldwork
We obtained specimens of the new species on nine mountains distributed across Sulawesi (Fig. 1). The sites where we captured the new species spanned a broad range of elevations, from ~500 m on Mts. Dako and Buliohuto to ~2,300 m on Mt. Latimojong. Sites where we did not find the new species tended to be lower-elevation areas. These comprised the lowest-elevation site near Mt. Gandang Dewata (~200 m); a small, geographically isolated mountain (1,550 m Mt. Tompotika, sampled at 350–600 m); and a large mountain where we only sampled relatively low-elevation areas (3,016 m Mt. Balease, sampled at 800–1,250 m). On Mt. Dako, we collected at two elevational bands, centered around 500 and 1,600 m. We found the new species in both areas. At the lower site, there were five syntopic shrew species comprising pale elongata, dark elongata, pale lea, C. nigripes, and C. rhoditis. At the high-elevation site we collected the new species, dark elongata, pale lea, dark lea, C. nigripes, and C. rhoditis.
Morphological evidence
Our initial examination of external morphology during the collecting expedition to Mt. Dako recognized only five species from the area. However, mitochondrial sequences suggested there were two species each contained among samples labeled C. elongata and C. lea. Upon reexamination of these specimens, we noted discrete color differences in the pelage and feet of these specimens that corresponded perfectly to the mitochondrial clades. We therefore moved forward with a working hypothesis that seven species occur on Mt. Dako (see colloquial names above).
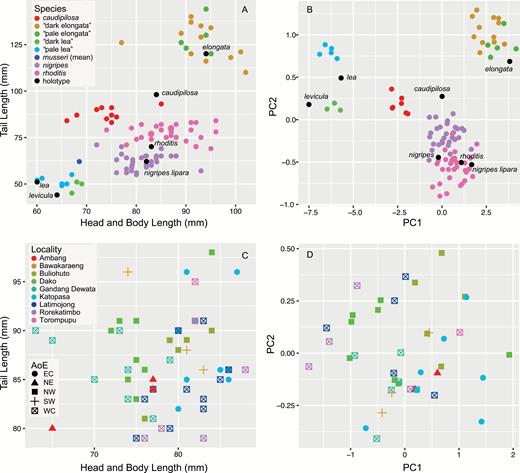
A bivariate plot of head and body length versus tail length that includes specimens from Mt. Dako and the holotypes of most species illustrates the phenotypic diversity of Sulawesi shrews (Fig. 2A). At one extreme, specimens of C. elongata have both long bodies and tails, while members of the new species and C. rhoditis are intermediate in both respects. Crocidura nigripes is intermediate in body size, but has a shorter tail. Crocidura lea, C. levicula, and C. musseri are all short-bodied and short-tailed. Hence, external measurements alone will distinguish the new species from C. elongata, C. lea, C. levicula, C. musseri, and C. nigripes, but it is proportionally similar to C. rhoditis. Our PCA of cranial variables from the holotypes of Sulawesi species and from specimens of all species from Mt. Dako also showed the new species to be distinct (Fig. 2B). The first component represented size and explained 92% of the variation, while the second component contrasted the breadth of the braincase with the length of the posterior part of the skull, but explained only 4.7% of the variation (Table 1). The new species is well separated along PC1 from C. elongata, C. lea, and C. levicula, but it is near C. nigripes along both vectors. Both analyses of morphometric variation among all samples of the new species show no discernible geographic pattern (Figs. 2C and 2D).
Loadings and variance from principal components analysis of 10 (all specimens from Mt. Dako) and 12 (new species only, sampled from across Sulawesi) cranial variables.
| Variable . | All Dako specimens . | . | New species only . | . |
|---|---|---|---|---|
| . | PC1 . | PC2 . | PC1 . | PC2 . |
| Condylo-incisive length | 0.724 | 0.166 | 0.71 | 0.187 |
| Brain breadth | 0.218 | −0.731 | 0.215 | −0.318 |
| Interorbital width | 0.119 | −0.136 | 0.112 | −0.262 |
| Rostral length | 0.310 | −0.19 | 0.343 | 0.344 |
| Post-palatal depth | 0.125 | 0.0 | 0.0 | −0.210 |
| Rostral width | 0.0 | 0.0 | 0.0 | −0.442 |
| Post-palatal length | 0.324 | 0.399 | 0.339 | −0.183 |
| Condyle to glenoid | 0.236 | 0.367 | 0.225 | −0.299 |
| Length upper toothrow | 0.319 | −0.169 | 0.295 | 0.328 |
| P4 to M3 | 0.178 | −0.235 | 0.134 | 0.0 |
| M2 to M2 | NA | NA | 0.177 | −0.319 |
| Palatal width | NA | NA | 0.0 | −0.313 |
| Standard deviation | 2.67 | 0.601 | 0.811 | 0.211 |
| Cumulative variance | 0.923 | 0.970 | 0.818 | 0.865 |
| Variable . | All Dako specimens . | . | New species only . | . |
|---|---|---|---|---|
| . | PC1 . | PC2 . | PC1 . | PC2 . |
| Condylo-incisive length | 0.724 | 0.166 | 0.71 | 0.187 |
| Brain breadth | 0.218 | −0.731 | 0.215 | −0.318 |
| Interorbital width | 0.119 | −0.136 | 0.112 | −0.262 |
| Rostral length | 0.310 | −0.19 | 0.343 | 0.344 |
| Post-palatal depth | 0.125 | 0.0 | 0.0 | −0.210 |
| Rostral width | 0.0 | 0.0 | 0.0 | −0.442 |
| Post-palatal length | 0.324 | 0.399 | 0.339 | −0.183 |
| Condyle to glenoid | 0.236 | 0.367 | 0.225 | −0.299 |
| Length upper toothrow | 0.319 | −0.169 | 0.295 | 0.328 |
| P4 to M3 | 0.178 | −0.235 | 0.134 | 0.0 |
| M2 to M2 | NA | NA | 0.177 | −0.319 |
| Palatal width | NA | NA | 0.0 | −0.313 |
| Standard deviation | 2.67 | 0.601 | 0.811 | 0.211 |
| Cumulative variance | 0.923 | 0.970 | 0.818 | 0.865 |
Loadings and variance from principal components analysis of 10 (all specimens from Mt. Dako) and 12 (new species only, sampled from across Sulawesi) cranial variables.
| Variable . | All Dako specimens . | . | New species only . | . |
|---|---|---|---|---|
| . | PC1 . | PC2 . | PC1 . | PC2 . |
| Condylo-incisive length | 0.724 | 0.166 | 0.71 | 0.187 |
| Brain breadth | 0.218 | −0.731 | 0.215 | −0.318 |
| Interorbital width | 0.119 | −0.136 | 0.112 | −0.262 |
| Rostral length | 0.310 | −0.19 | 0.343 | 0.344 |
| Post-palatal depth | 0.125 | 0.0 | 0.0 | −0.210 |
| Rostral width | 0.0 | 0.0 | 0.0 | −0.442 |
| Post-palatal length | 0.324 | 0.399 | 0.339 | −0.183 |
| Condyle to glenoid | 0.236 | 0.367 | 0.225 | −0.299 |
| Length upper toothrow | 0.319 | −0.169 | 0.295 | 0.328 |
| P4 to M3 | 0.178 | −0.235 | 0.134 | 0.0 |
| M2 to M2 | NA | NA | 0.177 | −0.319 |
| Palatal width | NA | NA | 0.0 | −0.313 |
| Standard deviation | 2.67 | 0.601 | 0.811 | 0.211 |
| Cumulative variance | 0.923 | 0.970 | 0.818 | 0.865 |
| Variable . | All Dako specimens . | . | New species only . | . |
|---|---|---|---|---|
| . | PC1 . | PC2 . | PC1 . | PC2 . |
| Condylo-incisive length | 0.724 | 0.166 | 0.71 | 0.187 |
| Brain breadth | 0.218 | −0.731 | 0.215 | −0.318 |
| Interorbital width | 0.119 | −0.136 | 0.112 | −0.262 |
| Rostral length | 0.310 | −0.19 | 0.343 | 0.344 |
| Post-palatal depth | 0.125 | 0.0 | 0.0 | −0.210 |
| Rostral width | 0.0 | 0.0 | 0.0 | −0.442 |
| Post-palatal length | 0.324 | 0.399 | 0.339 | −0.183 |
| Condyle to glenoid | 0.236 | 0.367 | 0.225 | −0.299 |
| Length upper toothrow | 0.319 | −0.169 | 0.295 | 0.328 |
| P4 to M3 | 0.178 | −0.235 | 0.134 | 0.0 |
| M2 to M2 | NA | NA | 0.177 | −0.319 |
| Palatal width | NA | NA | 0.0 | −0.313 |
| Standard deviation | 2.67 | 0.601 | 0.811 | 0.211 |
| Cumulative variance | 0.923 | 0.970 | 0.818 | 0.865 |
Inter- and intraspecific morphometric variation among Crocidura from Sulawesi. Upper plots show head and body length versus tail length (A) and first and second principal components from an analysis of 10 cranial dimensions (B) from all species of shrew known from Mt. Dako and the holotypes of all Crocidura species from Sulawesi, except for C. musseri. The mean values from the type series of C. musseri (taken from Ruedi 1995) are included in (A). No external measurements are available for the holotype of C. n. nigripes. Lower plots show geographic variation within C. caudipilosa sp. nov. for external measurements (C) and the first and second principal components from an analysis of 12 cranial dimensions (D) with individual points colored by locality and with area of endemism indicated by shape.
Genetic evidence
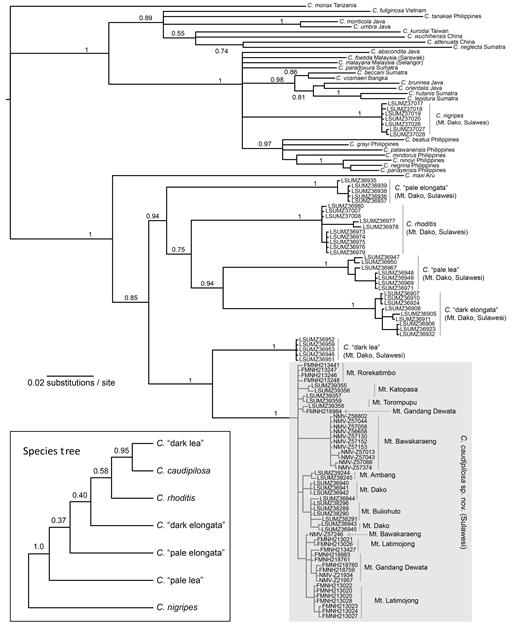
All new sequences were published by GenBank (accessions MK607621–MK607889 and MK645475–MK645545). Our mitochondrial alignment contains 715 characters (< 2% missing characters) and 120 individuals (93 new sequences from Sulawesi, including 50 of the new species). Following the PartitionFinder results, we divided the alignment by codon position and applied the SYM + I + Γ model to the first codon positions, the HKY + I model to the second positions, and the GTR + I + Γ model to the third positions. Our partitioned phylogenetic analysis of mitochondrial data appeared to converge quickly. Traces of the likelihood score plateaued in each run before 5 × 105 generations. As such, we discarded the first 106 generations, which left all parameters with per-run effective sample sizes > 500 (as estimated by MrBayes). Our resulting gene tree (Fig. 3) shows that all specimens of the new species form a clade, sister to dark lea. These species are both members of the endemic radiation of Sulawesi shrews that diversified in situ. This endemic radiation of six species is sister to C. maxi from Aru, a species also found on Java and several islands in the Lesser Sundas (Fig. 3; Kitchener et al 1994; Demos et al. 2017). Crocidura nigripes, the only known Sulawesi shrew that is not a member of this radiation, appears in a polytomy with several species from the Philippines and Sunda Shelf region. Among the nine mountains sampled for the new species, relatively little mitochondrial divergence (< 0.02 uncorrected p-distance) is evident (Fig. 3).
A Bayesian majority-rule consensus estimate of the mitochondrial gene tree of Southeast Asian Crocidura, including a new species from Sulawesi. Numbers at nodes are posterior probabilities (those below the species level or ≤ 0.7 are not shown). Tips are labeled with the specific epithet and their geographic provenance. Samples from Sulawesi are labeled with voucher numbers. Inset shows a species tree of all taxa sampled on Mt. Dako, Sulawesi Island, as estimated from eight nuclear loci using BPP.
Our nuclear alignments, when concatenated, contained 45 individuals of seven species (5–9 individuals per species) and 4,444 nucleotide positions, with 10% missing data. Our analyses of nuclear data, designed to test species limits and infer the species tree topology for samples collected on Mt. Dako, were entirely consistent across independent BPP runs and among prior settings. We inferred a posterior probability of 1.0 for every species postulated by mitochondrial and phenotypic patterns. In addition to the consistent delimitation model, the best tree always had the same topology. After fixing the species delimitation model to seven species, we reestimated the species tree using default BPP priors (Fig. 3). The topology strongly supports a sister relationship between the new species and dark lea, as in the mitochondrial gene tree, but otherwise, relationships within the endemic radiation (i.e., excluding C. nigripes) are poorly supported.
Taken together, this genetic and morphological evidence indicates that the specimens with hairy tails collected across Sulawesi represent a distinct species not previously described.
Crocidura caudipilosa, new species
Holotype
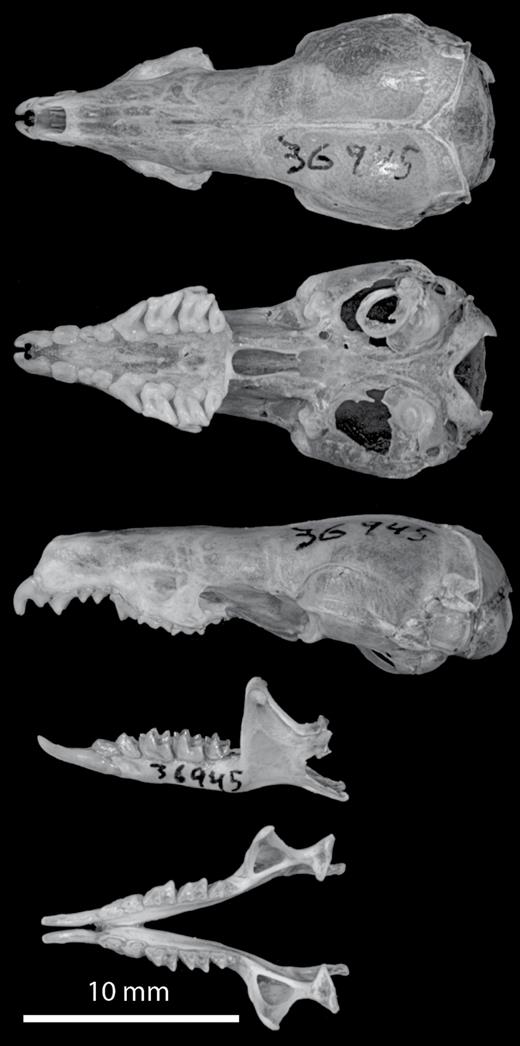
LSUMZ 36945, Fig. 4. An adult male collected on 8 March 2013 at 512 m above sea level on Mt. Dako by James L. Patton. The specimen was prepared as a traditional study skin, cleaned skull and skeleton, and frozen tissue. All parts are in excellent condition, but the left tympanic ring is separated from the skull. The holotype will be permanently curated at the Museum Zoologicum Bogoriense, Bogor, Indonesia, with catalog number MZB 41456.
Images of the skull and mandible of the holotype (LSUMZ 36945) of Crocidura caudipilosa sp. nov.
Paratypes
LSUMZ 36940–36944 and NMV C37330, C37551, C37304, C37305, C37267, all collected from ~1,600 m on Mt. Dako.
Other referred specimens
LSUMZ 39029, 39243–39246 from Mt. Ambang; NMV Z56658, Z56802, Z57013, Z57043, Z57044, Z57058, Z57066, Z57130, Z57152, Z57153, Z57246, and Z57374 from Mt. Bawakaraeng; LSUMZ 38289–38291, 38296, and NMV C37815 from Mt. Buliohuto; FMNH 218759–218762, 218983–218986, NMV Z21934, Z21957 from Mt. Gandang Dewata; LSUMZ 39355–39357, NMV Z61815, Z62413, Z62415, Z55561, Z55552, and Z62393 from Mt. Katopasa; FMNH 213020–213028, 213427, and NMV Z55257 from Mt. Latimojong; FMNH 213246–213248, 213441 from Mt. Rorekatimbo; and LSUMZ 39358–39359 from Mt. Torompupu.
Etymology
We combine the Latin caudi (tail) with pilosa (hairy), in recognition of the species’ most distinctive morphological trait. We recommend “Sulawesi hairy-tailed shrew” as an English common name.
Distribution
Crocidura caudipilosa is endemic to Sulawesi and widespread on the island. Specimens are known from the provinces of Central Sulawesi (Mts. Dako, Katopasa, Rorekatimbo, and Torompupu), Gorontalo (Mt. Buliohuto), West Sulawesi (Mt. Gandang Dewata), South Sulawesi (Mts. Bawakaraeng and Latimojong), and North Sulawesi (Mt. Ambang; Fig. 1).
Diagnosis
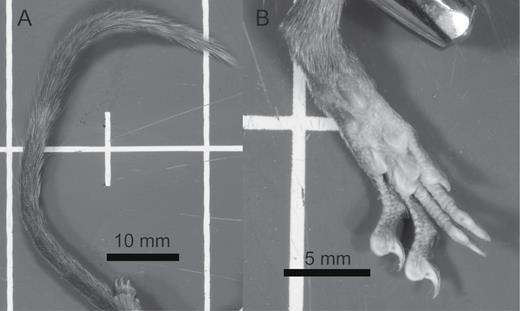
A slender shrew with a gray-brown dorsal pelage and silvery-gray venter (Fig. 5). The tail averages slightly longer than the head and body (Table 2) and is covered in applied hairs that are pigmented at the base, more so dorsally and proximally, giving the tail a dorsoventrally bicolored overall appearance, with a silvery tip. The tail also holds 7–10 mm long bristles along the proximal two-thirds to three-fourths of its length. Bristles decline in density and length distally. Combined, the applied hairs and bristles make the tail very hirsute (Fig. 6A). Dorsally the feet are brown, with paler digits. The interdigital pads are large and pale, matching the color of the digits (Fig. 6B). The skull is small and somewhat slender for a Crocidura, with rounded features. The dentition is only weakly developed. I3 is slightly larger than I2 in occlusal area.
Photograph of a live specimen (MZB 39852) of Crocidura caudipilosa sp. nov., captured on Mt. Katopasa.
Images showing the density of hair on the tail (A) and distribution of foot pads on the sole of the left hind foot (B) of Crocidura caudipilosa sp. nov. (LSUMZ 36944).
Description and comparisons
The pelage of the new species is gray-brown dorsally and slightly paler ventrally. Dorsal hairs are 4–7 mm long at mid-dorsum (individuals from higher elevations, e.g., > 2,000 m, appear to have longer pelages) and gray at the base, with a short brown tip. Ventral hairs are medium gray at the base, with a translucent tip, giving the pelage a slight silvery reflectance. Ventral hair lengths are similar to those on the dorsum. Crocidura rhoditis is similar in color to the new species and C. elongata varies from pale gray to a gray-brown that is similar to the color of the new species. All other Sulawesi Crocidura are darker than the new species, ranging from medium brown (C. levicula) to dark brown with a longer pelage (C. musseri), or dark gray to black (C. lea, C. nigripes).
The tail length averages 110% of head and body length, with most individuals falling between 100% and 120%. Other species known from Sulawesi have tails much longer than head and body (C. elongata: 120–163% on Mt. Dako), or shorter than head and body (C. lea, C. levicula, C. musseri, C. nigripes, and C. rhoditis; Fig. 2A). Most distinctively, the tail of the new species is covered in applied hairs along its entire length (Fig. 5, 6A). These approximately 2 mm long hairs are pigmented brown at their base, and the length of this pigmented section is greater on the dorsal and proximal portions of the tail. This gives the tail a slight bicolored appearance, with a brown dorsum and gray venter. The tip of the tail appears silvery from the pigment patterns in these hairs. No other Crocidura from Sulawesi, the Sunda region, or the Philippines has this great density of long, applied hairs along the tail, but some African species do have very hairy tails (e.g., C. turba). In the other species from Mt. Dako, the holotypes of five Sulawesi species, and the topotypical series of C. musseri, specimens have few to many applied hairs along the tail and they increase in length distally, but these hairs never approach the ~2 mm length seen in the new species. In many specimens of pale elongata and a small portion of dark elongata samples, the tail has a white tip ~15 mm long. However, the tails are uniformly colored along their length in the type series of C. elongata (USNM 217534, 217535) and other specimens referred to in the original description from Pinedapa, Middle Celebes (USNM 219448, 219449—Miller and Hollister 1921). Overall, the tails of C. lea, C. levicula, C. musseri, and C. nigripes are much darker than in the new species, while those of C. elongata and C. rhoditis are similar or slightly paler. The tail of the new species also holds sparse 7–10 mm long bristles along the proximal two-thirds to three-fourths of its length. Some bristles are brown at the base and translucent distally, whereas others are entirely translucent. Tail bristles are present to varying degrees on all other species of Crocidura known from Sulawesi, but their value as diagnostic characters may be limited by intraspecific variation (Ruedi 1995).
Dorsally, the feet are medium brown to dark brown, with silver highlights in the new species. The digits are paler than the foot. The claws are translucent and surrounded by a tuft of unpigmented hairs (Fig. 6B). The soles of the feet are scaly, as is typical of Crocidura (Hutterer et al. 2018), with large interdigital pads that fill the margin of the foot at the base of the digits. On the hind foot, the thenar pad is more prominent than the hypothenar, approaching twice the size in some specimens, and both pads are more pigmented posteriorly (Fig. 6B). On the forefoot, these pads are approximately equal in prominence. All foot pads and the ventral surfaces of the digits are paler than the surrounding, scaly sole. Other Sulawesi Crocidura have darker (C. lea, C. levicula, C. musseri, and C. nigripes) or paler feet (C. elongata and C. rhoditis). In C. musseri, although the pads are proportionately similar in size to those of the new species, they are darker than the surrounding sole of the foot. Dark lea has feet that are dark brown overall on both the dorsum and venter. On the dorsal surface, the integument is pale, but overlain with short, dark brown hairs. On the sole of the hind foot, the thenar is narrow, and less than twice the size of the hypothenar. In pale lea, the toes are white dorsally, but a mix of brown hairs on the foot gives a gray-brown, grizzled appearance that contrasts with the color of the toes. The pads on the sole of the foot are similar in proportion to those of dark lea, but overall, the color is paler in pale lea, with dark pigment showing only around the base of the thenar and hypothenar. In C. rhoditis, the hindfoot pads are similar in relative size, but the feet are much paler than in the new species, especially on the dorsum, where they are nearly white. In C. elongata, the feet are long and pale. The thenar is approximately twice as long as the hypothenar, but roughly equal in width. The hypothenar is lightly pigmented, but the thenar is pale like the sole of the foot. The feet of pale elongata are nearly white, similar to the pattern in C. rhoditis. In dark elongata, the dorsal surfaces of the feet are only slightly darker, with a mixture of brown and white hairs. As the name implies, C. nigripes has darker feet than most species. The dorsal surfaces are dark brown, due to pigment in both the skin and hairs. The soles of the feet are also brown, slightly paler than the dorsum, and the thenar and hypothenar pads are more nearly equal in size than in the new species. Pigment in these two pads is richest around the base, with a paler apex. Dorsally, the holotype of C. levicula has medium brown, uniformly colored feet that closely match the pelage. Ventrally, the pads are darker than the surrounding sole of the foot.
The face of the new species is typical of Crocidura. Mystacial vibrissae are brown proximally, unpigmented distally, and range from approximately 5 to 23 mm long, with the shorter whiskers having more anterior origins. A few short (~5 mm) interramal vibrissae emanate from the chin.
The skull is somewhat gracile for a Crocidura, with rounded features in dorsal view (Fig. 4). The lambdoid crest is present and more prominent than the minimally developed sagittal crest. The dorsal profile of the skull is relatively straight, but with a slight dip over the orbital region. From a dorsal view, the interorbital margins taper anteriorly, but this part of the skull is wide relative to overall skull size. The skulls of C. rhoditis and C. elongata are much larger than those of the new species, with C. elongata having a much longer skull, more angular shape in dorsal view, and a narrower, less-tapered interorbital region. Crocidura lea, C. levicula, and C. musseri skulls are much smaller. The skull of C. nigripes is similar in size to that of the new species, but with a more angular braincase.
The sinus canal, which may transmit the ophthalmic vein (McDowell 1958), forms a shallow arch along each side of the braincase. A small foramen lying about 1 mm below the canal, just posterior to the highest point of the arch, is connected to the main sinus by a branch in the canal. This conformation is similar in C. elongata, but in C. nigripes, there are 1–3 small foramina on each side, often with at least one lying directly on top of the main canal; in C. rhoditis there are either one large or two small foramina usually on, or very near, the main canal; in C. lea a single foramen lies just below or on the main canal, sometimes in a more anterior position than in the new species; and in C. levicula, the sinus canal is not smoothly arched, but rather descends above the foramen before rising again. Nevertheless, the sinus canal is distinctly above the foramen and no obvious branch connects them in C. levicula.
The antorbital bridge is wide relative to skull size in the new species, with a lachrymal foramen near the upper, anterior margin of the bridge. The antorbital bridges of C. lea, C. musseri, and C. nigripes are narrower, and in C. nigripes, the lachrymal foramen is placed lower on the bridge. The bridge is similarly broad in C. levicula.
From a ventral perspective, the palate is narrow and the dentition not particularly large in the new species. These are proportionately similar in C. elongata. The palates and dentitions of C. nigripes and C. rhoditis are wider and more robust. The palate of C. musseri is also wider than in the new species, but the dentition is not more strongly developed. I3 is slightly larger than I2 in the new species, C. elongata, C. levicula, C. rhoditis, and C. nigripes, but I3 is much larger than I2 in C. lea. The parastyle of P4 creates only a small point of contact between I3 and P4 in the new species. The breadth of contact between these teeth is greater in C. lea, similar in C. nigripes, but they do not make contact in C. levicula, C. rhoditis, or C. elongata. The parastyle of P4 is also narrower in C. elongata than in the new species. The lingual margins of M2 and M3 are relatively continuous in the new species, with the protocone and hypocone only modestly distinguishable. The protocone and hypocone are more distinct in the other species from Mt. Dako and in the holotypes of C. elongata, C. lea, C. nigripes, and C. rhoditis.
On the dentary, the coronoid forms a broad, nearly equilateral, upright triangle. That of C. nigripes is much narrower relative to its height, and that of C. rhoditis is slightly narrower, while in C. elongata, the overall shape is similar, but it is tilted slightly anterior. Other species have similarly shaped coronoids.
Ecology
The new species has a wide distribution on Sulawesi, and the low mitochondrial genetic distances across the island suggest that individuals have moved across the island relatively recently. The species also has a broad elevational range. On most of the mountains where we found it, C. caudipilosa was present at the full range of sampled elevations, which included both tropical lowland rainforest and moss forest sensu Musser (1987). We presume the species does not occur outside of forest, but trapping efforts in disturbed habitats have been limited.
Arboreality is sometimes suspected in shrew species with long tails (e.g., C. elongata, C. paradoxura, and C. dolichura—Ruedi 1995), which are thought to aid balancing on narrow branches. However, direct evidence of climbing in the wild is rarely available. In the case of C. caudipilosa, one specimen (NMV Z56802) was taken in a sticky trap intended for reptiles on a vertical tree trunk on Mt. Bawakaraeng. The animal was positioned facing down the trunk, approximately 1.5 m above the ground, suggesting it was descending from some position higher in the tree. The base of this tree was clear of branches and had only a light covering of moss, giving normally terrestrial animals few easy footholds. A second live individual (NMV Z62413), captured on Mt. Katopasa, was temporarily released and, without encouragement, it easily climbed a vertical tree trunk with a light covering of moss. These observations suggest that C. caudipilosa are skilled climbers and may routinely go up trees.
Discussion
Crocidura caudipilosa is readily distinguishable from all other known species of shrew in Southeast Asia by the length of applied hairs on the tail. The species also differs from other shrews on Sulawesi by a combination of external and cranial traits, such as body size, pelage and foot color, tail length, and the narrow palate and weak dentition. Genetic data support this conclusion and demonstrate that the new species is a member of the endemic radiation of Sulawesi shrews that now contains six described species and an unknown number of undescribed species (Ruedi et al. 1998; Esselstyn et al. 2009; Eldridge et al. 2018; this study). The simplicity of recognizing C. caudipilosa as a new species stands in stark contrast to the difficulty of identifying some of the shrew species described from Sulawesi nearly a century ago. In particular, we struggled to recognize which, if any, of four genetically and phenotypically distinguishable species from Mt. Dako were assignable to either C. lea or C. elongata. Ultimately, we were unable to clarify the issue and therefore used colloquial names for these four species.
Establishing a taxonomy that reflects both evolutionary history and extant diversity remains difficult in some groups of mammals, despite rapid progress in DNA sequencing technology (Rowe et al. 2011; Faircloth et al. 2012) and widespread adoption of integrative approaches to taxonomy (e.g., Fan et al. 2017; Rakotoarison et al. 2017; Hutterer et al. 2018). The challenge of reconciling taxonomy with evolutionary history remains an especially significant issue in groups that are diverse, small-bodied, and poorly represented in early natural history collections (e.g., Vespertilionidae, Soricidae). While small body size often makes the identification of diagnostic morphological characters difficult, the small sample sizes of some taxa that were available to many 19th- and early-20th-century mammalogists often led them to use very few specimens in their taxonomic treatments. Both factors have contributed to the generally poor resolution of shrew taxonomy. For example, among the five species of Crocidura described from Sulawesi by Miller and Hollister (1921), C. lea and C. levicula were represented by only the holotypes, and C. elongata was represented by only two specimens from the type locality and two more from a distant location. These and other descriptions lack a meaningful account of intraspecific variation. In this and other cases, widespread habitat loss further exacerbates the issue, with many type localities now devoid of natural habitat. For example, Temboan, North Sulawesi is the type locality for C. elongata, C. lea, C. nigripes, and C. rhoditis; it is a lowland area where the native forests have been heavily degraded, if not entirely razed. Extensive habitat conversion makes the potential for collection of new topotypes unlikely. This forces the modern taxonomist to attempt to assign specimens collected from different localities (often from higher elevation sites) to species defined without any concept of intraspecific variation, geographic or otherwise. As such, the best means of linking type specimens with modern collections is almost certainly by sequencing DNA obtained by destructively sampling the types. Prospects for doing so have improved dramatically over the last several years (Rowe et al. 2011; Ruane and Austin 2017); this may become a more common means of resolution, but museum curators are understandably cautious with type specimens.
The shrew fauna of Sulawesi is now known to contain at least nine species of Crocidura, seven of which are now described. The true number may be higher, depending for instance, on whether either of dark lea or pale lea is actually conspecific with C. lea and whether either pale elongata or dark elongata is conspecific with C. elongata. Whereas Eldridge et al. (2018), Ruedi and Vogel (1995), and Musser (1982, 1987) noted the possibility of C. elongata representing a complex of two or more species, our findings here further complicate the scenario by implying that specimens readily recognized previously by their long tail as C. elongata may not even be monophyletic (Fig. 3). Eldridge et al. (2018) showed that C. elongata has differentiated genetically among AoEs, among mountains, between high- and low-elevation forests across the island, and along individual elevational gradients, but they did not test the monophyly of C. elongata. At the time, this seemed unnecessary because the “species” is so morphologically distinctive. The extensive genetic diversity in C. elongata (Eldridge et al. 2018), with some of the pattern consistent with the widely reported AoE paradigm (e.g., Fooden 1969; Evans et al. 2003, 2008; Merker et al. 2009; Giarla et al. 2018), is a stark contrast to the minimal differentiation in mitochondrial DNA of C. caudipilosa. While it remains possible that nuclear genetic diversity could be partitioned by AoEs in the new species, we found no evidence of geographic differentiation in phenotypic traits (Figs. 3C and 3D).
The behavioral and ecological traits of the shrews in Sulawesi’s endemic radiation are almost entirely unknown, making it difficult to speculate how coexistence among these close relatives is maintained. Vertical separation is one possible axis of ecological differentiation among species. Both C. caudipilosa and C. elongata have long tails, a trait often associated with climbing ability in mammals, but also potentially indicating a saltatorial style of locomotion (Jenkins and Krause 1983; Rose 1987; Brosset 1988; Vaughan et al. 2015). Our field observations of climbing by C. caudipilosa suggest the species uses some resources above the ground, but we have no concept of how often or for what purpose these animals climb. The tail of C. caudipilosa is not extraordinarily long, nor are the feet unusual for a Crocidura, perhaps further suggesting that many species of shrew are more capable of climbing than is generally appreciated. Although most observations of arboreality in shrews are taken from captive animals (e.g., see Vogel 1974 for observations of Sylvisorex megalura), Heim de Balsac and Vuattoux (1969) captured an individual of Crocidura douceti 17 m above the ground and found a nest with unidentified juvenile shrews at 15 m height in the Côte d’Ivoire. Unfortunately, improving our understanding of how shrews use resources in trees will be difficult due to the lack of effective aboveground trapping techniques.
Clearly, the many remaining taxonomic issues in the Sulawesi radiation of Crocidura warrant a thorough systematic investigation. Although many issues are unclear, it is apparent that Sulawesi’s shrew fauna is unusual in that it is almost entirely derived from a single colonist ancestor. Only C. nigripes is not a member of this clade. Other islands in the region have either depauperate shrew faunas derived entirely from overwater colonization and allopatric speciation (e.g., the Philippine archipelago—Esselstyn et al. 2009; Giarla and Esselstyn 2015) or relatively diverse faunas derived from a combination of within-island and allopatric speciation (e.g., Java—Esselstyn et al. 2013; Demos et al. 2016). Insights on how speciation has proceeded in these differing contexts might improve our understanding of how coexistence occurs among shrew species that are superficially considered ecologically similar. However, ecological relations cannot be adequately understood until we have a taxonomy that reflects evolutionary history.
Summary statistics characterizing external and cranial dimensions (in mm) from specimens of Crocidura caudipilosa sp. nov.
| Variable . | Mean . | Range . | σ . | n . |
|---|---|---|---|---|
| Condylo-incisive length | 20.87 | 19.63–22.20 | 0.59 | 41 |
| Brain breadth | 9.48 | 9.00–9.99 | 0.23 | 41 |
| Interorbital width | 4.46 | 4.1–4.84 | 0.16 | 41 |
| Rostral length | 8.37 | 7.75–9.05 | 0.31 | 41 |
| Post-palatal depth | 3.78 | 3.56–4.05 | 0.13 | 41 |
| Rostral width | 2.92 | 2.67–3.22 | 0.15 | 41 |
| Post-palatal length | 9.50 | 8.83–10.24 | 0.30 | 41 |
| Condyle to glenoid | 8.27 | 7.89–8.76 | 0.21 | 41 |
| Length upper toothrow | 9.12 | 8.55–9.66 | 0.27 | 41 |
| P4 to M3 | 4.78 | 4.46–5.07 | 0.14 | 41 |
| M2 to M2 | 5.81 | 5.44–6.26 | 0.20 | 41 |
| Palatal width | 2.46 | 2.2–2.75 | 0.12 | 41 |
| Total length | 165.0 | 145–183 | 7.63 | 54 |
| Tail length | 86.6 | 79–98 | 4.69 | 53 |
| Hind foot lengtha | 16.8 | 15–19 | 0.99 | 53 |
| Ear length | 9.7 | 8–13 | 1.14 | 54 |
| Mass (g) | 8.9 | 6.5–12 | 1.18 | 53 |
| Variable . | Mean . | Range . | σ . | n . |
|---|---|---|---|---|
| Condylo-incisive length | 20.87 | 19.63–22.20 | 0.59 | 41 |
| Brain breadth | 9.48 | 9.00–9.99 | 0.23 | 41 |
| Interorbital width | 4.46 | 4.1–4.84 | 0.16 | 41 |
| Rostral length | 8.37 | 7.75–9.05 | 0.31 | 41 |
| Post-palatal depth | 3.78 | 3.56–4.05 | 0.13 | 41 |
| Rostral width | 2.92 | 2.67–3.22 | 0.15 | 41 |
| Post-palatal length | 9.50 | 8.83–10.24 | 0.30 | 41 |
| Condyle to glenoid | 8.27 | 7.89–8.76 | 0.21 | 41 |
| Length upper toothrow | 9.12 | 8.55–9.66 | 0.27 | 41 |
| P4 to M3 | 4.78 | 4.46–5.07 | 0.14 | 41 |
| M2 to M2 | 5.81 | 5.44–6.26 | 0.20 | 41 |
| Palatal width | 2.46 | 2.2–2.75 | 0.12 | 41 |
| Total length | 165.0 | 145–183 | 7.63 | 54 |
| Tail length | 86.6 | 79–98 | 4.69 | 53 |
| Hind foot lengtha | 16.8 | 15–19 | 0.99 | 53 |
| Ear length | 9.7 | 8–13 | 1.14 | 54 |
| Mass (g) | 8.9 | 6.5–12 | 1.18 | 53 |
a Includes the claws.
Summary statistics characterizing external and cranial dimensions (in mm) from specimens of Crocidura caudipilosa sp. nov.
| Variable . | Mean . | Range . | σ . | n . |
|---|---|---|---|---|
| Condylo-incisive length | 20.87 | 19.63–22.20 | 0.59 | 41 |
| Brain breadth | 9.48 | 9.00–9.99 | 0.23 | 41 |
| Interorbital width | 4.46 | 4.1–4.84 | 0.16 | 41 |
| Rostral length | 8.37 | 7.75–9.05 | 0.31 | 41 |
| Post-palatal depth | 3.78 | 3.56–4.05 | 0.13 | 41 |
| Rostral width | 2.92 | 2.67–3.22 | 0.15 | 41 |
| Post-palatal length | 9.50 | 8.83–10.24 | 0.30 | 41 |
| Condyle to glenoid | 8.27 | 7.89–8.76 | 0.21 | 41 |
| Length upper toothrow | 9.12 | 8.55–9.66 | 0.27 | 41 |
| P4 to M3 | 4.78 | 4.46–5.07 | 0.14 | 41 |
| M2 to M2 | 5.81 | 5.44–6.26 | 0.20 | 41 |
| Palatal width | 2.46 | 2.2–2.75 | 0.12 | 41 |
| Total length | 165.0 | 145–183 | 7.63 | 54 |
| Tail length | 86.6 | 79–98 | 4.69 | 53 |
| Hind foot lengtha | 16.8 | 15–19 | 0.99 | 53 |
| Ear length | 9.7 | 8–13 | 1.14 | 54 |
| Mass (g) | 8.9 | 6.5–12 | 1.18 | 53 |
| Variable . | Mean . | Range . | σ . | n . |
|---|---|---|---|---|
| Condylo-incisive length | 20.87 | 19.63–22.20 | 0.59 | 41 |
| Brain breadth | 9.48 | 9.00–9.99 | 0.23 | 41 |
| Interorbital width | 4.46 | 4.1–4.84 | 0.16 | 41 |
| Rostral length | 8.37 | 7.75–9.05 | 0.31 | 41 |
| Post-palatal depth | 3.78 | 3.56–4.05 | 0.13 | 41 |
| Rostral width | 2.92 | 2.67–3.22 | 0.15 | 41 |
| Post-palatal length | 9.50 | 8.83–10.24 | 0.30 | 41 |
| Condyle to glenoid | 8.27 | 7.89–8.76 | 0.21 | 41 |
| Length upper toothrow | 9.12 | 8.55–9.66 | 0.27 | 41 |
| P4 to M3 | 4.78 | 4.46–5.07 | 0.14 | 41 |
| M2 to M2 | 5.81 | 5.44–6.26 | 0.20 | 41 |
| Palatal width | 2.46 | 2.2–2.75 | 0.12 | 41 |
| Total length | 165.0 | 145–183 | 7.63 | 54 |
| Tail length | 86.6 | 79–98 | 4.69 | 53 |
| Hind foot lengtha | 16.8 | 15–19 | 0.99 | 53 |
| Ear length | 9.7 | 8–13 | 1.14 | 54 |
| Mass (g) | 8.9 | 6.5–12 | 1.18 | 53 |
a Includes the claws.
Nomenclatural statement: A Life Science Identifier (LSID) number was obtained for this publication: urn:lsid:zoobank.org:pub:7F61042D-EF07-4C5A-9A20-A70A5A7EF815
Acknowledgments
We thank M. Sarkam, M. Swanson, J. McGuire, T. Haryoko, J. Patton, and C. Patton for assistance with fieldwork and N. Woodman and two anonymous reviewers for helpful feedback. Funding was provided by the U.S. National Science Foundation (OISE-0965856, DEB-1343517, DEB-1441634, DEB-1457654, and DEB-1754393) and the National Geographic Society (9025-11; WW-160R-17). For facilitating access to voucher specimens, we thank L. Heaney, J. Phelps, and the late W. Stanley at FMNH, R. Voss and E. Westwig at AMNH, N. Woodman and D. Lunde at USNM, and C. Conroy at MVZ. JAE extends a special thank you to R. Timm for his extensive natural history expertise, keen editorial eye, infectious enthusiasm, and good-natured mentorship.
Literature Cited
American Society of Mammalogists.
Appendix I
Specimens examined
Crocidura caudipilosa: Mt. Dako: LSUMZ 36940–36945, NMV C37330, C37551, C37304, C37305, C37267; Mt. Latimojong: FMNH 213020–213028, 213427, NMV Z55257; Mt. Rorekatimbo: FMNH 213246–213248, 213441; Mt. Gandang Dewata: FMNH 218759–218762, 218983–218986, NMV Z21934, Z21957; Mt. Bawakaraeng: NMV Z56658, Z56802, Z57013, Z57043, Z57044, Z57058, Z57066, Z57130, Z57152, Z57153, Z57246, Z57374; Mt. Buliohuto: NMV C37815, LSUMZ 38289–38291, 38296; Mt. Katopasa: NMV Z55561, Z55552, Z61815, Z62393, Z62413, Z62415, LSUMZ 39355–39357; Mt. Torompupu: LSUMZ 39358–39359; Mt. Ambang: LSUMZ 39029, 39243–39246.
Crocidura elongata: Temboan, North Celebes: USNM 217534 (holotype), 217535.
Crocidura “pale elongata”: Mt. Dako: LSUMZ 36935–36939.
Crocidura “dark elongata”: Mt. Dako: LSUMZ 36905–36924, 36927–36930, 36932, 36933.
Crocidura “unknown elongata”: Pinedapa, Middle Celebes: USNM 219448, 219449.
Crocidura lea: Temboan, North Celebes: USNM 217553 (holotype).
Crocidura “pale lea”: Mt. Dako: LSUMZ 36947–36950, 36952, 36954–36956, 36958, 36960, 36964, 36967–36969, 36971.
Crocidura “dark lea”: Mt. Dako: LSUMZ 36946, 36951, 36953, 36957, 36959, 36961–36963, 36965, 36966.
Crocidura levicula: Pinedapa, Middle Celebes: USNM 219450 (holotype).
Crocidura musseri: Mt. Rorekatimbo: FMNH 213253–213255, 213259, 213263.
Crocidura nigripes: Temboan, North Celebes: USNM 217545 (holotype); Gimpoe, Middle Celebes: 219444 (holotype of C. n. lipara); Mamuju, West Sulawesi: FMNH 218703, 218704; Mt. Dako: LSUMZ 37017–37030, 37032, 37035–37049.
Crocidura rhoditis: Temboan, North Celebes: USNM 217550 (holotype); Mt. Dako: LSUMZ 36973–37016, 37031, 37033, 37034.