-
PDF
- Split View
-
Views
-
Cite
Cite
Meredith E Kernbach, Vincent M Cassone, Thomas R Unnasch, Lynn B Martin, Broad-spectrum light pollution suppresses melatonin and increases West Nile virus–induced mortality in House Sparrows (Passer domesticus), The Condor, Volume 122, Issue 3, 4 August 2020, duaa018, https://doi.org/10.1093/condor/duaa018
Close - Share Icon Share
Abstract
Artificial light at night (ALAN) has become a pervasive anthropogenic stressor for both humans and wildlife. Although many negative impacts of ALAN on human health have been identified, the consequences for infectious disease dynamics are largely unexplored. With the increase in popularity of energy efficient light-emitting diodes (LEDs), the effects of spectral composition of ALAN have also come into question. Previous studies showed that exposure to low levels of incandescent ALAN extended the infectious period of House Sparrows (Passer domesticus) infected with West Nile virus (WNV) without affecting mortality rates, thus increasing the pathogen initial reproductive rate (R0) by ~41%. Here, we asked whether exposure to broad-spectrum (3000 K [Kelvin; unit of color temperature]) ALAN suppressed melatonin, a hormone implicated in ALAN-induced physiological consequences, in House Sparrows. We then asked whether amber-hue bulbs (1800 K) could ameliorate the effects of WNV on individual sparrows, and whether broad-spectrum or blue-rich bulbs (3000 K and 5000 K, respectively) could exacerbate them. We found that exposure to low intensity (~5 lux) broad-spectrum (3000 K) ALAN significantly suppressed melatonin levels throughout the night. Second, we found that exposure to broad-spectrum and blue-rich (3000 + 5000 K) lights did not affect WNV viremia but did increase WNV-induced mortality. Conversely, birds exposed to amber-hue (1800 K) ALAN had lower viremia and mortality rates similar to controls (i.e. natural light conditions). This study demonstrates that ALAN affects melatonin regulation in birds, but this effect, as well as ALAN influences on infectious disease responses, can be ameliorated by particular lighting technologies.
Resumen
La luz artificial nocturna (LAN) se ha convertido en una fuente de estrés antropogénico ampliamente difundido tanto para los humanos como para la vida silvestre. Aunque se han identificado varios impactos negativos de la LAN en la salud de los humanos, las consecuencias para las dinámicas de las enfermedades infecciosas están muy poco exploradas. Con el aumento en popularidad de los diodos emisores de luz (LEDs, por sus siglas en inglés), de gran eficiencia energética, también han comenzado los cuestionamientos sobre los efectos de la composición espectral de la LAN. Estudios previos han demostrado que la exposición a bajos niveles de LAN incandescente extendió el período infeccioso en individuos de Passer domesticus infectados con el virus del Nilo Occidental (VNO) sin afectar las tasas de mortalidad, aumentando por ende la tasa reproductiva inicial del patógeno (R0) en ~41%. En este trabajo, analizamos si la exposición a la LAN de amplio espectro (3,000 K [Kelvin, unidad de temperatura del color]) suprimió en P. domesticus la melatonina, una hormona involucrada en las consecuencias fisiológicas inducidas por la LAN. Luego, nos preguntamos si las bombillas de color ámbar (1,800 K) podrían mejorar los efectos del VNO a nivel de individuo en P. domesticus, y si las bombillas de amplio espectro y de luz azul (3,000 K y 5,000 K, respectivamente) podrían exacerbarlos. Encontramos que la exposición a la LAN de amplio espectro (3,000 K) de alta intensidad (~5 lux) suprimió significativamente los niveles de melatonina a lo largo de la noche. Segundo, encontramos que la exposición a luces de amplio espectro y azul (3,000 + 5,000 K) no afectaron la viremia del VNO, pero si aumentaron la mortalidad inducida por el VNO. Por el contrario, las aves expuestas a la LAN de color ámbar (1,800 K) tuvieron tasas más bajas de viremia y mortalidad similares a los controles (i.e., condiciones de luz natural). Este estudio demuestra que la LAN afecta la regulación de melatonina en las aves, pero este efecto, al igual que la influencia de la LAN sobre la respuesta a las enfermedades infecciosas, puede ser mejorado por determinadas tecnologías de iluminación.
Introduction
Light pollution, especially artificial light at night (ALAN), is a widespread and influential anthropogenic stressor (Kyba et al. 2017). In addition to its prominence across busy highways and city centers, ALAN is found in rural greenspaces and along forest edges, many of which are occupied by wildlife (Longcore and Rich 2004). As the lightbulb was invented in the late 19th century, artificial nighttime lighting poses a relatively novel threat (Morison and Hughes 1991, Hedges and Kumar 2003). Alteration of light cycles, historically perhaps the most reliable environmental cue on Earth, can create mismatch and confusion in both circadian and circannual rhythms of the organisms exposed to such light pollution. Some of the best-known examples of these effects include earlier reproduction in passerines exposed to light pollution (Miller 2006, Dominoni et al. 2013) as well as the multiple dimensions of immune dysregulation observed in laboratory rodents (Bedrosian et al. 2011, Fonken et al. 2013). Light at night has also induced broad physiological consequences in birds such as hormonal dysregulation, shifted circadian regulation, and modified behavior (Schoech et al. 2013, Dominoni 2015, Alaasam et al. 2018). Nevertheless, the full extent to which these organismal effects translate into ecological consequences remains elusive (Kernbach et al. 2018).
Many effects of ALAN on animals are likely mediated by upstream circadian dysregulation of molecular timekeepers like melatonin. The brain’s suprachiasmatic nuclei (SCN), known as the central pacemaker, is responsible for coordinating circadian rhythms across the body. The pineal gland coordinates with the SCN, and integrates light cues from melanopsin receptors to encode time of day (Bell-Pedersen et al. 2005). Although the SCN and pineal gland are both important for maintaining daily rhythms, the pineal gland is responsible for the synthesis and secretion of the indoleamine hormone, melatonin (Hardeland et al. 2012). Melatonin, in addition to synchronizing molecular clocks throughout the body, aids many other physiological processes including gonadal development, inflammation control and antioxidant activity, thermoregulation, and metabolism (Yu and Reiter 1992, Cassone et al. 2009). Melatonin secretion is suppressed during exposure to light (i.e. day) and typically peaks during the middle of the dark phase (Blask et al. 2002). Indeed, exposure to light at night suppresses melatonin secretion in many birds and mammals, including humans (Yamada et al. 1988, Redlin 2001). Given the many roles that pineal-derived melatonin plays in the body, it is unsurprising that its suppression has been linked to multiple physiological consequences (i.e. mental disorders and immune dysregulation; Claustrat et al. 2005, Srinivasan et al. 2006, Castanon-Cervantes et al. 2010).
Some species affected by ALAN can serve as zoonotic reservoirs (i.e. hosts that naturally amplify pathogens that can spill into human populations). House Sparrow (Passer domesticus), American Robin (Turdus migratorius), Blue Jay (Cyanocitta cristata), and Northern Cardinal (Cardinalis cardinalis), for instance, are all important reservoirs of West Nile virus (WNV; Chace and Walsh 2006). They all also are quite common in ALAN-polluted habitats. Several bat species, too, are reservoirs of zoonoses, and these species alter their behavior when exposed to light at night (Calisher et al. 2006, Spoelstra et al. 2017). Mice in the genus Peromyscus and related rodents, hosts to Borrellia burgdorferi (the causative agent of Lyme disease) and hantavirus among other infections, also alter their nighttime behaviors and social interactions in the presence of light pollution (Han et al. 2015, Gaitan and Millien 2016, Hoffmann et al. 2018, 2019).
In a recent study, we found that House Sparrows exposed to a low intensity (5 lux) of broad-spectrum (3000 K) light at night (ALAN) maintained higher WNV titers for longer than controls; however, mortality rates to WNV were unaffected by ALAN (Kernbach et al. 2019). Using a traditional epidemiological framework (e.g., the comparison of the initial reproductive rate [R0] changes in response to an environmental change), we found that these modest effects on individual birds meant that ALAN might increase WNV R0 by as much as 41% (Wonham et al. 2004, Kernbach et al. 2019). Because ALAN has both organismal- and ecological-level effects, we felt it important to determine if changes in the practice of nighttime lighting could moderate some of these effects (Khan and Abas 2011). First, we wanted to determine if melatonin was dysregulated by broad-spectrum (3000 K) ALAN; if so, the blue-dominated spectra of these bulbs would be expected to blunt the natural circadian rhythms in melatonin. Melanopsin receptors are maximally sensitive to the blue-rich hues emitted by these light-emitting diodes (LEDs; Cashmore et al. 1999, Mure et al. 2007, Pawson and Bader 2014). To save money, many cities have begun switching to 1800 K, 3000 K, and 5000 K LED lighting, which is concerning because blue-rich hues are especially detrimental to both human and wildlife health (Falchi et al. 2011). Our second aim was to reveal whether blue-rich (5000 K) LED lighting exacerbates the effects of ALAN on avian WNV responses and whether other lighting options that mostly emit in the amber-hue (1800 K) part of the light spectrum could alleviate the impacts. These bulbs have had positive effects in some contexts (e.g., turtle nestling success), but their impacts in other ALAN contexts have been rarely investigated (Witherington and Bjorndal 1991, Ferenc and Leonard 2008, Gaston et al. 2012).
We focused on the House Sparrow, as ALAN affected competence of this species for WNV (Marra et al. 2004, Hanson et al. 2019). We studied WNV, a vector-transmitted arbovirus, because it continues to pose health threats to humans and wildlife across the United States (Centers for Disease Control and Prevention 2020). We predicted that exposure to broad-spectrum (3000 K) ALAN would suppress melatonin secretion and hinder viral resistance. For blue-rich (5000 K) ALAN, we expected even poorer resistance, allowing individuals to maintain high viral titers (and hence remain infectious) for longer periods of time (Wyse and Hazlerigg 2009). We also predicted that individuals exposed to amber-hue (1800 K) ALAN would manifest resistance comparable to controls (i.e. total darkness at night).
METHODS
Experimental Procedures
Melatonin experiment.
House Sparrows (n = 48) were captured in the Tampa Bay, Florida, area using mist nets during January, February, and March 2018. All birds were captured between 0530 and 0930 hours. Following capture, birds were transported to the University of South Florida (USF) campus vivarium and housed individually in 33 x 38 x 46 cm cages in visual and auditory proximity to one another under assigned lighting conditions (n = 24 control, n = 24 ALAN) for 2 weeks. Food (mixed seeds) and water were provided ad libitum throughout the study. All birds were housed under 12 hr of light and 12 hr of darkness (12L:12D) such that light during the simulated day period was ~150 lux emitted at 3000 K. At night, ALAN treatment birds were exposed to 5 lux of 3000 K light (i.e. the intensity emitted by a typical street lamp at night; Dominoni et al. 2013), whereas control birds were housed in near darkness (~0 lux). Spectral composition of light is measured in units of Kelvin (K) and describes the light appearance in commercially sold lightbulbs. Although typically thought of as heat intensity, Kelvin in this instance describes the color temperature.
Blood samples were collected twice prior to treatment exposure (i.e. on 2 separate nights) at 2000, 2200, 0000, 0200, and 0400 hours (Zeitgeber times 14, 16, 18, 20, 22). Individuals were only sampled once per night and at different timepoints between the 2 sampling nights to avoid anemia and any related adverse effects. Individuals were either exposed to ALAN or control conditions at night during the exposure duration of 2 weeks, and then sampled twice again using identical methods described above, for a total of 4 sampling nights. None of the individuals from this melatonin suppression study were used in the subsequent WNV-infection experiment; following the conclusion of this study, all individuals were euthanized.
The serum was extracted and stored at −40°C until samples were processed. Melatonin was quantified using a commercially available enzyme-linked immunosorbent assay (ELISA; RE54021, Tecan Industries, Switzerland). Samples ranged in volume from 70 to 110 µL and were diluted to 500 µL in volume for the assay parameters. Then, samples were treated according to the published Tecan Industries protocol, and melatonin concentrations were calculated for each volume of serum and extrapolated to pg mL−1 serum. This approach was validated by dilution of a separate set of sparrow serum samples collected from previous experiments performed in the Cassone lab to determine parallelism with the standard curve.
Spectral composition experiment.
House Sparrows (n = 71; not including individuals from the melatonin suppression study) were captured in the Tampa Bay area using mist nets during October and November 2018. All husbandry conditions were identical to those described above with the exception of light at night treatments. At night, control birds were housed in complete darkness (0 lux; n = 24) whereas all other groups were exposed to 5 lux (i.e. intensity emitted by a street lamp at night; Dominoni et al. 2013) of one of three forms of light: amber-hue (1800 K) LED light (SCS Exterior Wildlife and Habitat Lighting, catalogue no. GB030, certification no. 2018–057; n = 12), broad-spectrum (3000 K) LED light (n = 11), or blue-rich (5000 K) LED light (n = 24). The amber-hue bulb was selected from the Florida Fish and Wildlife Commission (FWC) Certified Wildlife Lighting list which is required by state law to be used in coastal communities (Florida Fish and Wildlife Conservation Commission 2019). Sample sizes were unequal due to housing limitations and the original study design (see Results). After the first 2 weeks, birds were transported from the USF animal biosafety-level 2 (ABSL-2) facility where they were originally housed upon capture to the USF animal biosafety-level 3 (ABSL-3) facility to be housed under the same lighting conditions as used prior to transfer to the new facility. Birds were placed inside bioBUBBLE secondary containment systems (bioBUBBLE, Fort Collins, Colorado, USA) within the ABSL-3 facility that enclosed bird cages to prevent aerosol circulation throughout the housing facility for the remainder of the study.
All birds were exposed to 101 plaque-forming units (PFUs) of New York 1999 strain (NY’99) WNV one day after transfer to BSL-3 (Kernbach et al. 2019). Following WNV exposure, blood samples were collected from birds on days 2, 4, 6, and 10, and serum was stored at −20°C until viral RNA extractions were performed. Body mass (to 0.1 g) was measured at WNV exposure and all sampling timepoints thereafter, and mortality was monitored daily. All birds were euthanized by isoflurane overdose followed by rapid decapitation on day 10 post-exposure. This study was also originally designed to capture whether supplementing melatonin could promote viral resistance in ALAN-exposed birds. Therefore, approximately one-half of all birds were administered 200 µg mL−1 crystalline melatonin dissolved in 0.5% EtOH in drinking water at night. As this treatment had no statistically significant effect on either treatment group (see Supplementary Material), this aspect of the experiment is not further addressed.
To quantify viremia, WNV RNA was first extracted from 10 µL of each frozen serum sample using the Qiagen QIAmp Viral Extraction Mini Kit (Qiagen Cat. No. 52906). WNV standards were also extracted from known concentrations stocks using the same methods. Following extractions, RNA was quantified using quantitative real-time polymerase chain reaction (qRT-PCR) using a one-step Taqman kit (iTaq Universal Probes One-Step Kit; Bio-Rad Catalogue No. 1725141). All samples were run in duplicate with negative controls to detect potential contamination. The stock titer was recently measured using plaque assays, and qPCR results closely mirrored plaque counts (Brien et al. 2013).
Data Analysis
Melatonin experiment.
Melatonin data were analyzed using a generalized linear model (GLM; gamma distribution) with the R base and car packages (Fox and Weisberg 2011). Melatonin concentration (pg mL−1) was the dependent variable and ALAN treatment and Zeitgeber time and their interaction were considered fixed effects. We also evaluated the GLM with a type III ANOVA to determine main effects. To confirm there were no pre-existing differences among treatment groups, we built a GLM to compare melatonin concentrations between treatment groups before exposure (Supplementary Material). To determine whether ALAN exposure affected melatonin concentrations, we compared the group to its initial concentration (pre-exposure and post-exposure; Supplementary Material), as well as the concentrations between groups following 2 weeks in their respective treatments (ALAN and control).
Spectral composition experiment.
Visual inspection of data indicated that 3000 K and 5000 K ALAN birds were quite similar in terms of viremia, body mass, and mortality rate. Statistical comparisons also indicated that these 2 groups were indistinguishable, so they were combined into a single “3000 + 5000 K ALAN” group for all other analyses (n = 35; see Supplementary Material). To evaluate effects of ALAN spectra (i.e. light type) on viremia, we modeled log10 transformed WNV titer as the dependent variable in a generalized linear mixed model (GLMM; distribution was non-normal) with light type (control, broad-spectrum/blue-rich [3000 + 5000 K] ALAN, and amber-hue [1800 K] ALAN), days-post exposure (2, 4, 6, and 10 dpe), and their interaction as fixed effects and bird ID as a random effect in RStudio using the lme4 package (Bates et al. 2015). We then also asked whether changes in body mass (% since exposure) were affected by lighting treatment, viremia, their interaction, and vigor (i.e. body mass prior to WNV exposure), again using GLMM in the RStudio lme4 package (Burgan et al. 2019).
To assess how birds tolerated infections (i.e. residual variation in performance at a given viremia), we calculated average viremia and average percent change in body mass for each individual across the course of its infection (Gervasi et al. 2017, Burgan et al. 2019). We plotted average viremia along the x-axis and average percent change body mass along the y-axis and used a linear regression to determine whether there was a relationship between the 2 variables.
Finally, to discern how ALAN spectra and other factors affected mortality rate, we used the survival, survminer, and dplyr packages for the Cox proportional hazards method in RStudio (Therneau and Lumley 2015, Wickham and Francois 2016, Kassambara 2018). We conducted 2 modeling exercises for WNV-dependent mortality. First, we checked whether ALAN spectra alone predicted mortality (with the body mass of an individual prior to infection as a covariate in the model). As body mass had no detectable effect it was excluded from further models (Supplementary Material). Our second Cox modelling effort incorporated average viremia and average percent change body mass from days 2 and 4 post-exposure as well as ALAN spectra and all 2- and 3-way interactions as potential predictors of mortality. We used only data from days 2 and 4 post-exposure here as no mortality occurred until after that period. Moreover, we were concerned that diminutions in health occurring late in the infection might confound our ability to detect drivers of mortality; in other words, we were interested to learn whether peak viremia or the nadir in body mass in response to infection predicted mortality. Other studies have taken a similar approach for similar reasons (Gervasi et al. 2017). To determine whether individuals died earlier or later than expected, we compared residual variation in days until death based on average viremia (days 2 and 4) between treatment groups by conducting a one-way ANOVA followed by a Tukey pairwise comparison to more easily visualize the underlying differences in mortality.
RESULTS
Exposure to ALAN Suppresses Melatonin in House Sparrows
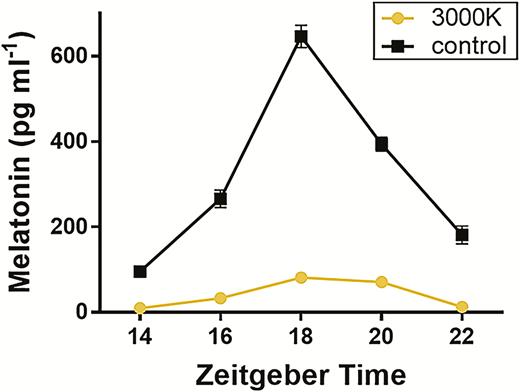
We found that exposure to 3000 K ALAN significantly suppressed melatonin concentrations at night (ALAN*Exposure χ 2 = 186.419, P = < 2e−16; Figure 1). Additionally, the melatonin concentrations within the ALAN-exposed groups were significantly suppressed from pre- to post-exposure timepoints (χ 2 = 7.7698, P = 0.005). Finally, we confirmed that there were no pre-existing differences in melatonin secretion among the 2 groups (χ 2 = 0.0225, P = 0.881).
Melatonin concentration (pg mL−1) measured during the dark phase at Zeitgeber times 14 (2000 hours), 16 (2200 hours), 18 (0000 hours), 20 (0200 hours), and 22 (0400 hours) in both control (~0 lux, darkness; n = 24) and 3000 K ALAN exposed (n = 24) birds post-exposure/captivity.
Amber-hue ALAN Enhances West Nile Virus Resistance
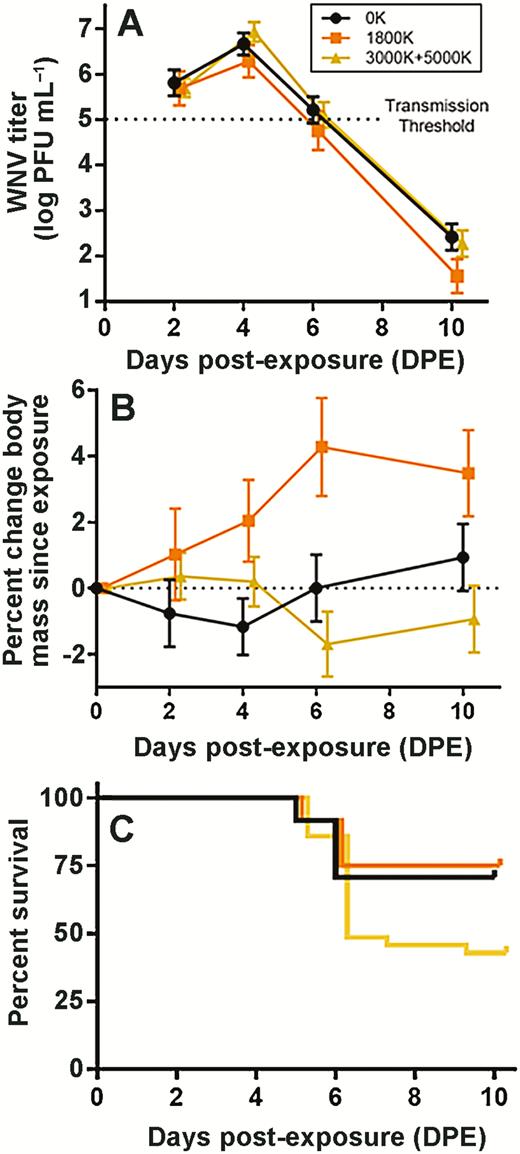
We detected a significant main effect of light type (χ 2 = 6.9942, P = 0.030) on WNV viremia (Figure 2A). We found that the difference was driven by significantly lower viremia in the amber-hue ALAN treatment group (1800 K, t = 2.530, P = 0.011; Table 1), which persisted across the entire post-exposure period (1800 K * day linear, t = 2.724, P = 0.006; 1800 K * day quadratic, t = 1.999, P = 0.046). Viremia did not differ between broad-spectrum/blue-rich ALAN and control groups however (3000 + 5000 K, t = 0.290, P = 0.772). Light type, day, and their interaction did not affect percent change in body mass since WNV exposure, but there was a significant interaction between 3000 + 5000 K ALAN and day (3000 K + 5000 K * day linear, t = 2.071, P = 0.038), such that these birds lost more body mass than the other groups since WNV exposure (Figure 2B).
Summary statistics of the generalized linear mixed model to determine effects of day, treatment, and their interaction on WNV viremia accounting for random effects of bird ID. A type III ANOVA revealed the main effects of both day and treatment had an effect on WNV viremia. Upon further investigation of the GLMM output, we determined that these main effects are driven by significantly lower viremia in the amber-hue (1800 K) ALAN exposed group and the interaction between day (linear (lin) and quadratic (quad)) and the amber-hue group. Significant terms are in bold.
| ANOVA (Type III) Parameter . | χ 2 value . | df . | P value . | . |
|---|---|---|---|---|
| Day | 76.3587 | 3 | < 2e−16 | |
| Treatment | 6.9942 | 2 | 0.03029 | |
| Day * Treatment | 8.7008 | 6 | 0.19111 | |
| GLMM Fixed effects Parameter | Estimate | SE | T value | P value |
| Day (linear) | 0.101127 | 0.012972 | 7.796 | 6.41e−15 |
| Day (quadratic) | 0.073140 | 0.010849 | 6.742 | 1.57e−11 |
| Day (cubic) | 0.014650 | 0.008698 | 1.684 | 0.09212 |
| 1800 K treatment | 0.031555 | 0.012474 | 2.530 | 0.01142 |
| 3000/5000 K treatment | 0.002687 | 0.009257 | 0.290 | 0.77165 |
| Day (lin) * 1800 K | 0.067238 | 0.024680 | 2.724 | 0.00644 |
| Day (quad) * 1800 K | 0.041429 | 0.020729 | 1.999 | 0.04565 |
| Day (cub) * 1800 K | 0.015780 | 0.016147 | 0.977 | 0.32842 |
| Day (lin) * 3000/5000 K | 0.006164 | 0.018069 | 0.341 | 0.73300 |
| Day (quad) * 3000/5000 K | 0.006026 | 0.015218 | 0.396 | 0.69214 |
| Day (cub) * 3000/5000 K | −0.003912 | 0.011954 | −0.327 | 0.74351 |
| ANOVA (Type III) Parameter . | χ 2 value . | df . | P value . | . |
|---|---|---|---|---|
| Day | 76.3587 | 3 | < 2e−16 | |
| Treatment | 6.9942 | 2 | 0.03029 | |
| Day * Treatment | 8.7008 | 6 | 0.19111 | |
| GLMM Fixed effects Parameter | Estimate | SE | T value | P value |
| Day (linear) | 0.101127 | 0.012972 | 7.796 | 6.41e−15 |
| Day (quadratic) | 0.073140 | 0.010849 | 6.742 | 1.57e−11 |
| Day (cubic) | 0.014650 | 0.008698 | 1.684 | 0.09212 |
| 1800 K treatment | 0.031555 | 0.012474 | 2.530 | 0.01142 |
| 3000/5000 K treatment | 0.002687 | 0.009257 | 0.290 | 0.77165 |
| Day (lin) * 1800 K | 0.067238 | 0.024680 | 2.724 | 0.00644 |
| Day (quad) * 1800 K | 0.041429 | 0.020729 | 1.999 | 0.04565 |
| Day (cub) * 1800 K | 0.015780 | 0.016147 | 0.977 | 0.32842 |
| Day (lin) * 3000/5000 K | 0.006164 | 0.018069 | 0.341 | 0.73300 |
| Day (quad) * 3000/5000 K | 0.006026 | 0.015218 | 0.396 | 0.69214 |
| Day (cub) * 3000/5000 K | −0.003912 | 0.011954 | −0.327 | 0.74351 |
Summary statistics of the generalized linear mixed model to determine effects of day, treatment, and their interaction on WNV viremia accounting for random effects of bird ID. A type III ANOVA revealed the main effects of both day and treatment had an effect on WNV viremia. Upon further investigation of the GLMM output, we determined that these main effects are driven by significantly lower viremia in the amber-hue (1800 K) ALAN exposed group and the interaction between day (linear (lin) and quadratic (quad)) and the amber-hue group. Significant terms are in bold.
| ANOVA (Type III) Parameter . | χ 2 value . | df . | P value . | . |
|---|---|---|---|---|
| Day | 76.3587 | 3 | < 2e−16 | |
| Treatment | 6.9942 | 2 | 0.03029 | |
| Day * Treatment | 8.7008 | 6 | 0.19111 | |
| GLMM Fixed effects Parameter | Estimate | SE | T value | P value |
| Day (linear) | 0.101127 | 0.012972 | 7.796 | 6.41e−15 |
| Day (quadratic) | 0.073140 | 0.010849 | 6.742 | 1.57e−11 |
| Day (cubic) | 0.014650 | 0.008698 | 1.684 | 0.09212 |
| 1800 K treatment | 0.031555 | 0.012474 | 2.530 | 0.01142 |
| 3000/5000 K treatment | 0.002687 | 0.009257 | 0.290 | 0.77165 |
| Day (lin) * 1800 K | 0.067238 | 0.024680 | 2.724 | 0.00644 |
| Day (quad) * 1800 K | 0.041429 | 0.020729 | 1.999 | 0.04565 |
| Day (cub) * 1800 K | 0.015780 | 0.016147 | 0.977 | 0.32842 |
| Day (lin) * 3000/5000 K | 0.006164 | 0.018069 | 0.341 | 0.73300 |
| Day (quad) * 3000/5000 K | 0.006026 | 0.015218 | 0.396 | 0.69214 |
| Day (cub) * 3000/5000 K | −0.003912 | 0.011954 | −0.327 | 0.74351 |
| ANOVA (Type III) Parameter . | χ 2 value . | df . | P value . | . |
|---|---|---|---|---|
| Day | 76.3587 | 3 | < 2e−16 | |
| Treatment | 6.9942 | 2 | 0.03029 | |
| Day * Treatment | 8.7008 | 6 | 0.19111 | |
| GLMM Fixed effects Parameter | Estimate | SE | T value | P value |
| Day (linear) | 0.101127 | 0.012972 | 7.796 | 6.41e−15 |
| Day (quadratic) | 0.073140 | 0.010849 | 6.742 | 1.57e−11 |
| Day (cubic) | 0.014650 | 0.008698 | 1.684 | 0.09212 |
| 1800 K treatment | 0.031555 | 0.012474 | 2.530 | 0.01142 |
| 3000/5000 K treatment | 0.002687 | 0.009257 | 0.290 | 0.77165 |
| Day (lin) * 1800 K | 0.067238 | 0.024680 | 2.724 | 0.00644 |
| Day (quad) * 1800 K | 0.041429 | 0.020729 | 1.999 | 0.04565 |
| Day (cub) * 1800 K | 0.015780 | 0.016147 | 0.977 | 0.32842 |
| Day (lin) * 3000/5000 K | 0.006164 | 0.018069 | 0.341 | 0.73300 |
| Day (quad) * 3000/5000 K | 0.006026 | 0.015218 | 0.396 | 0.69214 |
| Day (cub) * 3000/5000 K | −0.003912 | 0.011954 | −0.327 | 0.74351 |
The effects of spectral composition of ALAN on (A) WNV viremia (log10 PFU mL−1), (B) percent change body mass since exposure (g), and (C) percent survival. Birds exposed to amber-hue (1800 K) ALAN (n = 12) had significantly lower viremia throughout the course of infection and significant interaction across days post-exposure when day is described as both a linear and quadratic function (A). There are no main effects of treatment or day on percent change body mass; however, there is a significant interaction between broad-spectrum and blue-rich (3000 K + 5000 K) ALAN (n = 35) and day when day is modeled as a linear function (B). 3000 + 5000 K ALAN exposed birds incurred a significantly mortality rate than control (n = 24) and amber-hue exposed birds, which is partly driven by average viremia on days 2 and 4 post-exposure (C).
Broad-spectrum ALAN Increases WNV-induced Mortality
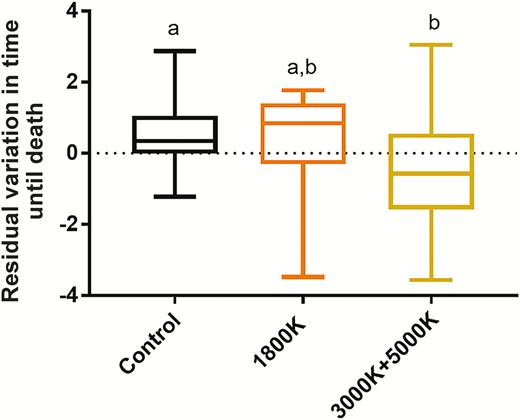
For tolerance, we found that there was no relationship between average viremia and average percent change body mass (R2 = 0.015, P = 0.316). Therefore, we focused on percent change in body mass since WNV exposure, independent of viremia, as a proxy of health status during infection for further analyses. We found that light type alone affected survival rate post-WNV exposure, driven by significantly higher mortality in the broad-spectrum/blue-rich ALAN group (χ 2 = 6.0217, P = 0.049; 3000 + 5000 K, z = 1.893, P = 0.0583; Figure 2C). Additionally, both average viremia and average percent change in body mass affected mortality (Table 2); higher titer and greater mass loss exacerbated mortality risk. A type III ANOVA revealed that main effects of light type, average percent change body mass, average viremia, the interaction between average viremia and treatment, and the interaction between average percent change body mass and average viremia all had significant effects on mortality. In other words, broad-spectrum ALAN mortality rates to WNV remained significantly higher even after the addition of other parameters, meaning that light type alone explained differences in mortality rates (z = 2.205, P = 0.027). Additionally, average viremia from days 2 and 4 (z = 3.079, P = 0.002) and the interaction between average viremia and treatment (z = −2.189, P = 0.029) were significant predictors of mortality. When we calculated the residual variation in time (days) until death based on an individual’s average viremia, we revealed a significant difference between light treatments (F = 3.561, df = 2, P = 0.034): broad-spectrum/blue-rich ALAN exposed birds died on average approximately 1 day earlier than expected compared to controls (adjusted P = 0.035; Figure 3).
Summary statistics of the Cox proportional-hazards analysis to determine effects of treatment, average percent change body mass, average viremia, and their interaction on mortality. A type III ANOVA revealed that all bolded parameters are significant predictors of mortality including treatment, average percent change in body mass since exposure (days 2 and 4), average viremia (days 2 and 4), the interaction between treatment and average viremia, and the interaction between average change on body mass and average viremia. The model outputs illustrated which parameters are driving the significant main effects observed, including significantly higher mortality in the broad-spectrum (3000 + 5000 K) ALAN group and average viremia as a strong indicator of future mortality. Interestingly, the interaction between the broad-spectrum ALAN group and average viremia is significant, indicating that the relationship between viremia and days until death is different in that group. Significant terms are in bold.
| ANOVA (Type III) Parameter . | χ 2 Value . | df . | P value . | . |
|---|---|---|---|---|
| Treatment | 7.3586 | 2 | 0.02524 | |
| Avg % Δ Mass | 4.0220 | 1 | 0.04491 | |
| Avg Viremia | 15.2721 | 1 | 9.308e−05 | |
| Treat * % Δ Mass | 3.9696 | 2 | 0.13741 | |
| Treat * Avg Viremia | 6.5208 | 2 | 0.03837 | |
| % Δ Mass * Avg Viremia | 4.3120 | 1 | 0.03785 | |
| Treat * % Δ Mass * Viremia | 4.1748 | 2 | 0.12401 | |
| Cox Prop-Hazards Model Parameter | Coefficient | SE | Z score | P value |
| 1800 K Treatment (2) | 1.557e + 01 | 9.729e + 00 | 1.601 | 0.10943 |
| 3000 + 5000 K Treatment (3) | 1.950e + 01 | 8.843e + 00 | 2.205 | 0.02743 |
| Avg % Δ Mass | −5.311e + 00 | 2.932e + 00 | −1.811 | 0.07014 |
| Avg Viremia | 3.577e + 00 | 1.162e + 00 | 3.079 | 0.00208 |
| Treat (2) * Avg % Δ Mass | 2.685e + 00 | 5.821e + 00 | 0.461 | 0.64465 |
| Treat (3) * Avg % Δ Mass | 5.158e + 00 | 2.946e + 00 | 1.751 | 0.07993 |
| Treat (2) * Avg Viremia | −2.028e + 00 | 1.313e + 00 | −1.544 | 0.12260 |
| Treat (3) * Avg Viremia | −2.561e + 00 | 1.170e + 00 | −2.189 | 0.02863 |
| % Δ Mass * Avg Viremia | 7.055e-01 | 3.776e−01 | 1.868 | 0.06172 |
| Treat (2) * Avg % Δ Mass * Avg Viremia | −3.491e-01 | 8.921e−01 | −0.391 | 0.69558 |
| Treat (3) * Avg % Δ Mass * Avg Viremia | −6.864e-01 | 3.796e−01 | −1.809 | 0.07052 |
| ANOVA (Type III) Parameter . | χ 2 Value . | df . | P value . | . |
|---|---|---|---|---|
| Treatment | 7.3586 | 2 | 0.02524 | |
| Avg % Δ Mass | 4.0220 | 1 | 0.04491 | |
| Avg Viremia | 15.2721 | 1 | 9.308e−05 | |
| Treat * % Δ Mass | 3.9696 | 2 | 0.13741 | |
| Treat * Avg Viremia | 6.5208 | 2 | 0.03837 | |
| % Δ Mass * Avg Viremia | 4.3120 | 1 | 0.03785 | |
| Treat * % Δ Mass * Viremia | 4.1748 | 2 | 0.12401 | |
| Cox Prop-Hazards Model Parameter | Coefficient | SE | Z score | P value |
| 1800 K Treatment (2) | 1.557e + 01 | 9.729e + 00 | 1.601 | 0.10943 |
| 3000 + 5000 K Treatment (3) | 1.950e + 01 | 8.843e + 00 | 2.205 | 0.02743 |
| Avg % Δ Mass | −5.311e + 00 | 2.932e + 00 | −1.811 | 0.07014 |
| Avg Viremia | 3.577e + 00 | 1.162e + 00 | 3.079 | 0.00208 |
| Treat (2) * Avg % Δ Mass | 2.685e + 00 | 5.821e + 00 | 0.461 | 0.64465 |
| Treat (3) * Avg % Δ Mass | 5.158e + 00 | 2.946e + 00 | 1.751 | 0.07993 |
| Treat (2) * Avg Viremia | −2.028e + 00 | 1.313e + 00 | −1.544 | 0.12260 |
| Treat (3) * Avg Viremia | −2.561e + 00 | 1.170e + 00 | −2.189 | 0.02863 |
| % Δ Mass * Avg Viremia | 7.055e-01 | 3.776e−01 | 1.868 | 0.06172 |
| Treat (2) * Avg % Δ Mass * Avg Viremia | −3.491e-01 | 8.921e−01 | −0.391 | 0.69558 |
| Treat (3) * Avg % Δ Mass * Avg Viremia | −6.864e-01 | 3.796e−01 | −1.809 | 0.07052 |
Summary statistics of the Cox proportional-hazards analysis to determine effects of treatment, average percent change body mass, average viremia, and their interaction on mortality. A type III ANOVA revealed that all bolded parameters are significant predictors of mortality including treatment, average percent change in body mass since exposure (days 2 and 4), average viremia (days 2 and 4), the interaction between treatment and average viremia, and the interaction between average change on body mass and average viremia. The model outputs illustrated which parameters are driving the significant main effects observed, including significantly higher mortality in the broad-spectrum (3000 + 5000 K) ALAN group and average viremia as a strong indicator of future mortality. Interestingly, the interaction between the broad-spectrum ALAN group and average viremia is significant, indicating that the relationship between viremia and days until death is different in that group. Significant terms are in bold.
| ANOVA (Type III) Parameter . | χ 2 Value . | df . | P value . | . |
|---|---|---|---|---|
| Treatment | 7.3586 | 2 | 0.02524 | |
| Avg % Δ Mass | 4.0220 | 1 | 0.04491 | |
| Avg Viremia | 15.2721 | 1 | 9.308e−05 | |
| Treat * % Δ Mass | 3.9696 | 2 | 0.13741 | |
| Treat * Avg Viremia | 6.5208 | 2 | 0.03837 | |
| % Δ Mass * Avg Viremia | 4.3120 | 1 | 0.03785 | |
| Treat * % Δ Mass * Viremia | 4.1748 | 2 | 0.12401 | |
| Cox Prop-Hazards Model Parameter | Coefficient | SE | Z score | P value |
| 1800 K Treatment (2) | 1.557e + 01 | 9.729e + 00 | 1.601 | 0.10943 |
| 3000 + 5000 K Treatment (3) | 1.950e + 01 | 8.843e + 00 | 2.205 | 0.02743 |
| Avg % Δ Mass | −5.311e + 00 | 2.932e + 00 | −1.811 | 0.07014 |
| Avg Viremia | 3.577e + 00 | 1.162e + 00 | 3.079 | 0.00208 |
| Treat (2) * Avg % Δ Mass | 2.685e + 00 | 5.821e + 00 | 0.461 | 0.64465 |
| Treat (3) * Avg % Δ Mass | 5.158e + 00 | 2.946e + 00 | 1.751 | 0.07993 |
| Treat (2) * Avg Viremia | −2.028e + 00 | 1.313e + 00 | −1.544 | 0.12260 |
| Treat (3) * Avg Viremia | −2.561e + 00 | 1.170e + 00 | −2.189 | 0.02863 |
| % Δ Mass * Avg Viremia | 7.055e-01 | 3.776e−01 | 1.868 | 0.06172 |
| Treat (2) * Avg % Δ Mass * Avg Viremia | −3.491e-01 | 8.921e−01 | −0.391 | 0.69558 |
| Treat (3) * Avg % Δ Mass * Avg Viremia | −6.864e-01 | 3.796e−01 | −1.809 | 0.07052 |
| ANOVA (Type III) Parameter . | χ 2 Value . | df . | P value . | . |
|---|---|---|---|---|
| Treatment | 7.3586 | 2 | 0.02524 | |
| Avg % Δ Mass | 4.0220 | 1 | 0.04491 | |
| Avg Viremia | 15.2721 | 1 | 9.308e−05 | |
| Treat * % Δ Mass | 3.9696 | 2 | 0.13741 | |
| Treat * Avg Viremia | 6.5208 | 2 | 0.03837 | |
| % Δ Mass * Avg Viremia | 4.3120 | 1 | 0.03785 | |
| Treat * % Δ Mass * Viremia | 4.1748 | 2 | 0.12401 | |
| Cox Prop-Hazards Model Parameter | Coefficient | SE | Z score | P value |
| 1800 K Treatment (2) | 1.557e + 01 | 9.729e + 00 | 1.601 | 0.10943 |
| 3000 + 5000 K Treatment (3) | 1.950e + 01 | 8.843e + 00 | 2.205 | 0.02743 |
| Avg % Δ Mass | −5.311e + 00 | 2.932e + 00 | −1.811 | 0.07014 |
| Avg Viremia | 3.577e + 00 | 1.162e + 00 | 3.079 | 0.00208 |
| Treat (2) * Avg % Δ Mass | 2.685e + 00 | 5.821e + 00 | 0.461 | 0.64465 |
| Treat (3) * Avg % Δ Mass | 5.158e + 00 | 2.946e + 00 | 1.751 | 0.07993 |
| Treat (2) * Avg Viremia | −2.028e + 00 | 1.313e + 00 | −1.544 | 0.12260 |
| Treat (3) * Avg Viremia | −2.561e + 00 | 1.170e + 00 | −2.189 | 0.02863 |
| % Δ Mass * Avg Viremia | 7.055e-01 | 3.776e−01 | 1.868 | 0.06172 |
| Treat (2) * Avg % Δ Mass * Avg Viremia | −3.491e-01 | 8.921e−01 | −0.391 | 0.69558 |
| Treat (3) * Avg % Δ Mass * Avg Viremia | −6.864e-01 | 3.796e−01 | −1.809 | 0.07052 |
Residual variation of the means of time (days) until death as a function of average viremia during days 2 and 4 post-exposure. A one-way ANOVA analysis followed by a Tukey pairwise comparison showed that there was a significant difference between broad-spectrum (3000 + 5000 K) ALAN (n = 35) and control (n = 24) birds (P = 0.034). The significant difference exists between control (a) and broad-spectrum (b) treatments; amber-hue treatment (a,b; n = 12) did not differ from either control (a) or broad-spectrum treatments (b).
DISCUSSION
Light type affected how House Sparrows coped with WNV infections. Broad-spectrum (3000 K) ALAN suppressed melatonin throughout the night after only 2 weeks of exposure. Alternatively, exposure to amber-hue ALAN (1800 K) increased WNV resistance by maintaining lower WNV burdens for shorter periods of time and perhaps reduced competence to transmit to vectors (Sears et al. 2011, Burgan et al. 2018). Broad-spectrum/blue-rich ALAN (3000 + 5000 K) exposure, however, did not reduce viral resistance, as seen previously, but it did increase WNV-induced mortality rates. Additionally, individuals exposed to the broad-spectrum/blue-rich ALAN died from WNV infection at lower viral burdens than control individuals. Altogether, our data indicate that the type of ALAN to which organisms are exposed likely affects melatonin secretion and could exacerbate or ameliorate how zoonotic diseases affect populations. Below we discuss both the organismal and ecological ramifications of our results as well as the mitigation opportunities they present in the interest of wildlife and human health.
Melatonin Actions on Antiviral Immune Defenses
Exposure to low intensities of 3000 K ALAN is enough to significantly suppress melatonin concentrations at night. Melatonin, as emphasized above, plays many active roles in the body including coordinating and controlling immune defenses. Early research discovered a close relationship between the fluctuations of melatonin and differentiation of granulocytes (i.e. toxic-granulated white blood cells), suggesting that melatonin played a key role in synchronizing immunological processes throughout the body (Kuci et al. 1988). Since then, many other relationships between the rhythmicity and concentration of melatonin and immunity have been discovered, including effects important for antiviral defenses. For example, elevating melatonin concentrations in birds at night increases heterophil activity, which is important for recognizing and engulfing apoptotic or virally infected cells (Rodríguez et al. 1999, Kogut et al. 2005, Uematsu and Akira 2006). Although phagocytes (i.e. white blood cells that engulf and destroy virally infected cells) can generate a disproportionate amount of free radicals, melatonin also protects against potential oxidative damage by acting as an antioxidant itself (Reiter 1996, Babior 2000, Galano et al. 2011). In other words, the collateral damage that phagocytic antiviral responses generate via radical oxygen species can typically be attenuated by melatonin directly. Furthermore, melatonin induces an anti-apoptotic regulation of T cells during differentiation, which undoubtedly affects the identification and elimination of virally infected cells by CD4 + and CD8 + lymphocytes (Sainz et al. 1995). It is therefore reasonable to expect that melatonin suppression via ALAN might mediate how House Sparrows control WNV infection. Some studies revealed that melatonin suppression via light at night exposure can propagate a Th1-biased humoral response, which is typically inflammatory in nature, and when uncontrolled, can cause immunopathology (Carrillo-Vico et al. 2013). Indeed, in human neuroinvasive WNV cases, polarized T-cell responses are associated with increased pathogenesis (James et al. 2016). Blocking melatonin actions in the pineal gland via propranolol (a melatonin receptor antagonist) suppressed multiple aspects of cellular and humoral immunity (Claustrat et al. 2005).
ALAN Exposure, Viral Resistance, and Its Relationship to Melatonin
In a previous study, we found multiple indications that antiviral immune defenses and damage attenuation were affected in WNV-infected House Sparrows exposed to low-intensity 3000 K ALAN (Kernbach et al. 2019). A weighted gene correlation network analysis (WGCNA) identified multiple networks of closely co-regulated genes that were differentially expressed between ALAN and control individuals. One network containing many aspects of the anti-WNV immune pathway, including the hub (i.e. most highly connected) gene, OASL, was upregulated sooner during infection in ALAN-exposed than controls (Mashimo et al. 2002, Tag-El-Din-Hassan et al. 2012, Kernbach et al. 2019). Although OASL stimulates antiviral responses, its advanced upregulation suggests that WNV infection disseminated more quickly in individuals that were exposed to ALAN (Choi et al. 2015). Dissemination of virus can be accelerated with the presence of reactive oxygen species; these reactive oxygen species are often neutralized by melatonin, which may be one mechanism whereby suppression of melatonin expedited viremia (Bonilla et al. 2004). Furthermore, a different network containing the hub gene, TRAP1 (heat shock protein [HSP] 75), was upregulated later during infection, when viremia in ALAN-exposed individuals was very high (Kernbach et al. 2019). TRAP1 is often upregulated in response to excessive oxidative stress, which might become common in the absence of melatonin (Bonilla et al. 2004, Hua et al. 2007). It has been suggested that TRAP1 and other heat shock proteins also aid the cellular entry of flaviviruses (i.e. a class of positive-sense and single-stranded RNA viruses; Rastogi et al. 2016). In this light, TRAP1 might be upregulated to compensate for an absence of melatonin, and in the process, allow for enhanced viral replication by aiding cellular entry or inhibiting apoptosis of infected cells (Hua et al. 2007).
ALAN Exposure, Immunopathology, Mortality, and a Potential Role for Melatonin
Furthermore, the WGCNA analysis from our previous study revealed that individuals exposed to ALAN were also experiencing severe immunopathology. Another network upregulated by ALAN-exposed individuals contained 2 hub genes, ATP11B and PLBD1 (Kernbach et al. 2019). ATP11B is associated with sepsis, an extreme response of the immune system that can cause life-threatening organ damage (Hu 2013). Similarly, PLBD1 is expressed during severe malaria infection and exposure to oxidative or thermal stressors (Chovatiya and Medzhitov 2014, Sobota et al. 2016). A handful of studies have explored the relationship between melatonin and sepsis, including the administration of melatonin as a potential therapeutic (Şener et al. 2005, Galley et al. 2014). The antioxidant and anti-inflammatory properties of melatonin contribute to the control of collateral damage during an immune response. As above, melatonin suppression might have led to the septic symptoms we observed in ALAN-exposed individuals. In support, the administration of melatonin to WNV-infected mice reduced the risk of encephalitis and resulting mortality (Ben-Nathan et al. 1995). Additionally, mice infected with Venezuelan equine encephalitis virus (VEEV) experienced similar protective benefits from melatonin supplementation with effects ranging from enhanced viral resistance (signified by lower viremia burdens) to decreased risk of mortality (Bonilla et al. 1997). Here, we observed that House Sparrows exposed to broad-spectrum ALAN experienced higher WNV-induced mortality rates and succumbed to infection at lower viral burdens than their control counterparts. Although we cannot directly attribute these effects to melatonin suppression, we encourage follow-up work to determine whether melatonin suppression is a mechanism by which light pollution induces increased mortality risk.
Melatonin Independent Effects of ALAN Exposure on Immune Defenses
In this study, we observed that exposure to amber-hue ALAN slightly, yet significantly, reduced WNV burden. Although we make a case that melatonin suppression partly mediates the effects of light at night exposure on antiviral immunity, we do not anticipate that circulating melatonin concentrations differed between control and amber-hue ALAN exposed individuals. Exposure to ALAN increases nighttime activity levels in captive birds and foraging opportunity in wild shorebirds (Dwyer et al. 2013, Alaasam et al. 2018). Perhaps amber-hue nocturnal illumination provided the opportunity for individuals to forage at night to offset the costs associated with melatonin suppression. Resource availability and quality are linked to immune function; therefore, the link between food intake, metabolism, and immunity should be investigated in individuals exposed to ALAN (Lochmiller et al. 1993, Siva-Jothy and Thompson 2002).
Alternatively, enhanced immune defenses may be related to an evolutionary association between nighttime illumination and risk of injury in prey species. Risk of predation for several organisms is highest during brightly lit nights including especially the full moon phase (Daly et al. 1992, Prugh and Golden 2014). Some nocturnally active rodents avoid illuminated areas including moonlight, where predation risk is high (Lima 1998, Upham and Hafner 2013). Perhaps exposure to light at night initiates antipredator responses, which mobilize and activate of aspects of the immune system in anticipation of injury (Martin 2009). Indeed, predation risk is associated with exaggerated immune responses in insect species (Duong and McCauley 2016). The association between nocturnal illumination and predation risk may contribute to the enhanced immune responses and lower WNV burdens we observed in here for amber-hue ALAN exposed birds.
Seasonality in ALAN Effects on WNV Resistance and Mortality
When comparing the present study to our previous findings, we discovered a large seasonal difference in WNV responses. When individuals were exposed to broad-spectrum ALAN during spring months, individuals maintain significantly higher WNV burdens for longer without incurring increased mortality rates (Kernbach et al. 2019). However, in this study focusing on birds caught in fall, individuals exposed to broad-spectrum ALAN showed no significant differences in WNV viremia but did incur a higher WNV-induced mortality rate. There are several different phenomena that may explain these seasonal effects.
Organisms naturally possess seasonal patterns in the properties of their immune system (Nelson and Demas 1996). These fluctuations may correlate with the risk of exposure during different times of year, such as influenza during winter or arboviruses during late summer (Dowell 2001, Blackmore et al. 2003). Additionally, immunity is often traded off for other important fitness investments during energetically demanding life history stages such as reproduction, migration, and molt (Sheldon and Verhulst 1996, Martin et al. 2008). Other stressful or unpredictable conditions such as resource shortage, habitat loss, or extreme weather events may force individuals to reroute energy intended for immune defenses toward other processes required for immediate survival (Lochmiller and Deerenberg 2000, Dobson 2009). A further investigation of the effects of ALAN on host competence over the annual cycle would both enhance our understanding of how light pollution alters infectious disease dynamics and creates an opportunity to predict when and where exposure risk is highest (Wonham et al. 2004, Rushing et al. 2017). Although we are unsure what contexts drive seasonal patterns in WNV competence, the effects of ALAN across seasons likely affects within-individual dynamics and ecological level outcomes.
Alternative Lighting Options
Here, we found that exposure to amber-hue LED lightbulbs alleviated most, if not all, negative effects we observed of ALAN exposure on WNV infection results in House Sparrows. This suggests that amber-hue light types may be a viable alternative to other LED lighting methods. Indeed, energy-efficient LED lightbulbs in this spectra are commercially available in multiple models (Florida Fish and Wildlife Conservation Commission 2019). Because these bulbs are already widely used along shorelines, advocating for their use within neighborhoods, cities, roadways, and other developed areas with nighttime illumination should not be met by harsh criticism (Ferenc and Leonard 2008). Although less feasible, lights-out programs advocated by organizations such as the International Dark-Sky Association would likely also be beneficial to wild organisms. In both this and our previous studies, we found that control birds who are housed in near-complete darkness do not incur as much WNV-induced mortality or damage (Kernbach et al. 2019). Other studies have recognized the benefit of lights-out programs such as decreasing building collision rates in nocturnally migrating birds (Winger et al. 2019).
Other studies have suggested the benefits of alternative lighting (such as the technology we used in this study) for other wildlife taxa as well. The use of amber-hue filtered light at night is less impactful on several species of non-passerine wildlife such as green turtles (Chelonia mydas), loggerhead turtles (Caretta caretta), and Newell’s Shearwater (Puffinus newelli) (Ferenc and Leonard 2008, Longcore et al. 2018). Furthermore, installation of amber-hue lighting should also have benefits for other organisms including humans. Indeed, exposure to blue-rich light at night can negatively affect sleep quality, thermoregulation, and resting heart rate in humans, all of which can be alleviated by shifting the spectral composition of nighttime lighting (Cajochen et al. 2005, Chellappa et al. 2013). Therefore, we advocate switching to amber-hue LED lightbulbs as this alternative still offers energy-efficient benefits while eliminating many health risks associate with blue-rich light exposure.
CONCLUSION
Our experiment confirmed that melatonin was significantly suppressed by low-intensity broad-spectrum (3000 K) light in House Sparrows. These data provide a plausible link between light at night exposure and antiviral immune dysregulation. However, more work needs to be done to directly link melatonin suppression to the aspects of anti-WNV immunity detailed above. We tested whether altering the spectral composition of ALAN would affect WNV resistance and mortality; broad-spectrum ALAN enhanced WNV-induced mortality and amber-hue ALAN slightly enhanced viral resistance while maintaining low rates of mortality relative to controls. These results suggest that the substitution of blue-rich wavelengths with amber-hue light at night might be a viable alternative to limiting negative consequences. We stress that ALAN has the potential to impact other parameters that influence WNV transmission and outbreak potential such as vector biting rate, WNV extrinsic incubation period, and other parameters, all of which need to be considered when describing infectious disease dynamics (Kernbach et al. 2018). Furthermore, it is important to consider other parameters that are known to impact infectious disease outbreak (e.g., area of impervious surfaces, human population density, climate) to determine where to focus intervention efforts. Although our findings here suggest viable lighting alternatives exist, it is still important to consider how spectral composition of ALAN affects other factors important to WNV transmission before recommending blanket lightbulb switches across the nation.
ACKNOWLEDGMENTS
We thank Haley Hanson, Kyle Koller, Bilal Koussayer, Sarah Guzinski, and Samantha Oakey for input on the experimental design and experimental execution. We also thank Erik Hofmeister for sharing the WNV NY’99 strain.
Funding statement: We thank USF College of Public Health and NSF 1257773 for funding.
Ethics Statement: All experimental work was pre-approved by and performed according to IACUC #2716 and IBC #1323.
Author contributions: M. E. Kernbach contributed to the design and execution of the experiment, data collection, data analysis, and manuscript writing. V. M. Cassone contributed to the design of the melatonin administration. T. R. Unnasch contributed to the design of BSL3 work and funding. L. B. Martin contributed to the design and execution of the experiment, data analysis, manuscript revision, and funding.
Data depository: Analyses reported in this article can be reproduced using the data provided by Kernbach et al. (2020).
Conflict of interest statement: We declare that we have no competing interests.