Groundwater Quality Assessment in a Volcanic Mountain Range (South of Gran Canaria Island, Spain)
Abstract
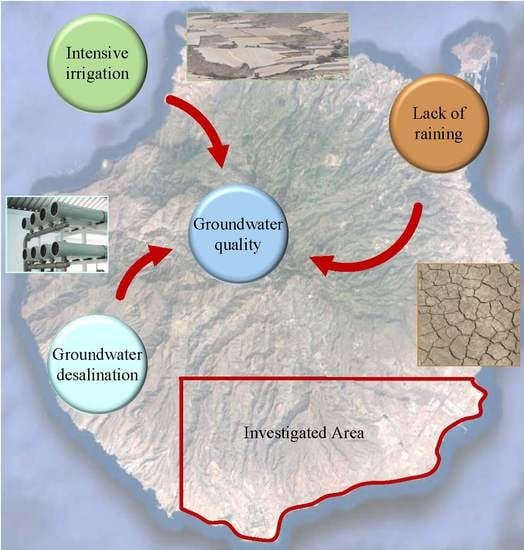
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sampling and Analytics
3. Results and Discussions
3.1. Waters of Type I (Cl-Ca)
3.2. Waters of Type II (Mg-Cl, Na-SO4)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Custodio, E. Aquifer overexploitation: What does it mean? Hydrogeol. J. 2002, 10, 254–277. [Google Scholar] [CrossRef]
- Serio, F.; Miglietta, P.P.; Lamastra, L.; Ficocelli, S.; Intini, F.; De Leo, F.; De Donno, A. Groundwater nitrate contamination and agricultural land use: A grey water footprint perspective in Southern Apulia Region (Italy). Sci. Total Environ. 2018, 645, 1425–1431. [Google Scholar] [CrossRef]
- Klassen, J.; Allen, D.M. Assessing the risk of saltwater intrusion in coastal aquifers. J. Hydrol. 2017, 551, 730–745. [Google Scholar] [CrossRef]
- Liu, S.; Tang, Z.; Gao, M.; Hou, G. Evolutionary process of saline–water intrusion in Holocene and Late Pleistocene groundwater in southern Laizhou Bay. Sci. Total Environ. 2017, 607–608, 586–599. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, B.; Shi, L.; Ye, Y. Dynamic variation characteristics of seawater intrusion in underground water-Sealed oil Storage cavern under island tidal environment. Water 2019, 11, 130. [Google Scholar] [CrossRef]
- Kløve, B.; Ala-Aho, P.; Bertrand, G.; Gurdak, J.J.; Kupfersberger, H.; Kværner, J.; Muotka, T.; Mykrä, H.; Preda, E.; Rossi, P.; et al. Climate change impacts on groundwater and dependent ecosystems. J. Hydrol. 2014, 518, 250–266. [Google Scholar] [CrossRef]
- Chun, J.A.; Lim, C.; Kim, D.; Kim, J.S. Assessing impacts of climate change and sea-level rise on seawater intrusion in a coastal aquifer. Water 2018, 10, 357. [Google Scholar] [CrossRef]
- Van Vliet, M.T.H.; Zwolsman, J.J.G. Impact of summer droughts on the water quality of the Meuse river. J. Hydrol. 2008, 353, 1–17. [Google Scholar] [CrossRef]
- Chunn, D.; Faramarzi, M.; Smerdon, B.; Alessi, D.S. Application of an integrated SWAT–MODFLOW Model to evaluate potential impacts of climate change and water withdrawals on groundwater–surface water interactions in West–Central Alberta. Water 2019, 11, 110. [Google Scholar] [CrossRef]
- Ruiz-García, A.; Feo-García, J. Estimation of maximum water recovery in RO desalination for different feedwater inorganic compositions. Desalin. Water Treat. 2017, 70, 34–45. [Google Scholar] [CrossRef]
- Custodio, E. Low permeability volcanics in the Canary Islands (Spain). In Proceedings of the International Congress on Hydrology of Rocks of Low Permeability, Tucson, AZ, USA, 7–12 January 1985. [Google Scholar]
- Custodio, E.; del Carmen Cabrera, M.; Poncela, R.; Puga, L.-O.; Skupien, E.; del Villar, A. Groundwater intensive exploitation and mining in Gran Canaria and Tenerife, Canary Islands, Spain: Hydrogeological, environmental, economic and social aspects. Sci. Total Environ. 2016, 557–558, 425–437. [Google Scholar] [CrossRef] [PubMed]
- Guerra Marrero, J.L. The market of the water in Canarias. In Underground Waters and Urban Supply (El Mercado del Agua en Canarias. Aguas Subterráneas y Abastecimiento Urbano); Geological and Mining Institute of Spain (IGME): Madrid, Spain, 2000. (In Spanish) [Google Scholar]
- Spanish Statistical Office (INE). Review of the Municipal Register of Inhabitants (Revisión del Padrón Municipal de Habitantes); Spanish Statistical Office (INE): Madrid, Spain, 2017. [Google Scholar]
- IGME. ITGE Geological Map of Spain, Island of Gran Canaria (Mapa Geológico de España, Isla de Gran Canaria); Geological and Mining Institute of Spain (IGME): Madrid, Spain, 1992. [Google Scholar]
- Herrera, C. Use of the relationship Cl/Br like tracer hydrogeochemical in underground hydrology (Utilización de la relación Cl-Br como trazador hidroquímico en hidrología subterránea). Bol. Geol. Min. 2000, 111, 49–68. [Google Scholar]
- Lomoschitz, A.; Meco, J.; Corominas, J. The Barranco de Tirajana basin, Gran Canaria (Spain). A major erosive landform caused by large landslides. Geomorphology 2002, 42, 117–130. [Google Scholar] [CrossRef]
- Rosen, M.R. Sedimentologic and geochemical constraints on the evolution of Bristol Dry Lake Basin, California, U.S.A. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1991, 84, 229–257. [Google Scholar] [CrossRef]
- Nurmi, P.A.; Kukkonen, I.T.; Lahermo, P.W. Geochemistry and origin of saline groundwaters in the Fennoscandian Shield. Appl. Geochem. 1988, 3, 185–203. [Google Scholar] [CrossRef]
- Nishri, A.; Stiller, M.; Rimmer, A.; Geifman, Y.; Krom, M. Lake Kinneret (The Sea of Galilee): The effects of diversion of external salinity sources and the probable chemical composition of the internal salinity sources. Chem. Geol. 1999, 158, 37–52. [Google Scholar] [CrossRef]
- Al-Bassam, A.M.; Al-Rumikhani, Y.A. Integrated hydrochemical method of water quality assessment for irrigation in arid areas: Application to the Jilh aquifer, Saudi Arabia. J. Afr. Earth Sci. 2003, 36, 345–356. [Google Scholar] [CrossRef]
- Mallick, J.; Singh, C.K.; AlMesfer, M.K.; Kumar, A.; Khan, R.A.; Islam, S.; Rahman, A. Hydro-geochemical assessment of groundwater quality in Aseer Region, Saudi Arabia. Water 2018, 10, 1847. [Google Scholar] [CrossRef]
- Hadi, K.M.B. Evaluation of the suitability of groundwater quality for reverse osmosis desalination. Desalination 2002, 142, 209–219. [Google Scholar] [CrossRef]
- Minissale, A.; Magro, G.; Vaselli, O.; Verrucchi, C.; Perticone, I. Geochemistry of water and gas discharges from the Mt. Amiata silicic complex and surrounding areas (central Italy). J. Volcanol. Geotherm. Res. 1997, 79, 223–251. [Google Scholar] [CrossRef]
- Yang, N.; Wang, G.; Shi, Z.; Zhao, D.; Jiang, W.; Guo, L.; Liao, F.; Zhou, P. Application of multiple approaches to investigate the hydrochemistry evolution of groundwater in an arid region: Nomhon, Northwestern China. Water 2018, 10, 1667. [Google Scholar] [CrossRef]
- Aleem, M.; Shun, C.J.; Li, C.; Aslam, A.M.; Yang, W.; Nawaz, M.I.; Ahmed, W.S.; Buttar, N.A. Evaluation of groundwater quality in the vicinity of Khurrianwala Industrial Zone, Pakistan. Water 2018, 10, 1321. [Google Scholar] [CrossRef]
- Galy, A.; France-Lanord, C. Weathering processes in the Ganges–Brahmaputra basin and the riverine alkalinity budget. Chem. Geol. 1999, 159, 31–60. [Google Scholar] [CrossRef]
- Cendrero, A.; Sánchez, J.; Antolin, C.; Arnal, S.; Diaz de Terán, J.R.; Francés, E.; Martínez, V.; Moiunõ, M.; Nieto, M.; Nogales, I.; et al. Geoscientific maps for planning in semi–arid regions: Valencia and Gran Canaria, Spain. Eng. Geol. 1990, 29, 291–319. [Google Scholar] [CrossRef]
- Cruz-Fuentes, T.; Cabrera Mdel, C.; Heredia, J.; Custodio, E. Groundwater salinity and hydrochemical processes in the volcano–sedimentary aquifer of La Aldea, Gran Canaria, Canary Islands, Spain. Sci. Total Environ. 2014, 484, 154–166. [Google Scholar] [CrossRef]
- Consejo Insular de Aguas de Gran Canaria. Hydrological Plan of Gran Canaria; Consejo Insular de Aguas de Gran Canaria: Gran Canaria, Spain, 2010. [Google Scholar]
- Ruiz-García, A.; Feo-García, J. Antiscalant cost and maximum water recovery in reverse osmosis for different inorganic composition of groundwater. Desalin. Water Treat. 2017, 73, 46–53. [Google Scholar] [CrossRef]
- Gasparini, A. Hydrochimie et Géochimie Isotopique de Circulations Souterraines en Milieu Volcanique sous Climat Semi–Aride (Grande Canarie, Iles Canaries). Ph.D. Thesis, Université de Paris Sud., Paris, France, 1989. (In French). [Google Scholar]
- Naranjo, G.; Cruz–Fuentes, T.; Cabrera, D.M.; Custodio, E. Estimating natural recharge by means of chloride mass balance in a volcanic aquifer: Northeastern Gran Canaria (Canary Islands, Spain). Water 2015, 7, 2555–2574. [Google Scholar] [CrossRef]
- Custodio, E.; Badiella, P. Partial Report of Amurga–1 (Informe Parcial Amurga–1. Evaluación de datos de Bombeo de los Pozos de Amurga). Private report. 1990. (In Spanish) [Google Scholar]
- Custodio, E.C.J. Partial Report of Amurga–2 (Informe Parcial Amurga–2. Recopilación y Síntesis de los datos Químicos de los Pozos de Amurga). Private report. 1993. (In Spanish) [Google Scholar]
- Gonfiantini, R.; Simonot, M. Isotopic investigation of groundwater in the Cul–de–Sac Plain, Haiti. In Isotope Techniques in Water Resources Development; International Atomic Energy Agency: Vienna, Austria, 1987; Available online: https://inis.iaea.org/collection/NCLCollectionStore/_Public/19/054/19054614.pdf?r=1&r=1. (accessed on 10 April 2019).
- Lloyd, J.W.; Miles, J.C. An examination of the mechanisms controlling groundwater gradients in hyper–arid regional sedimentary basins1. JAWRA J. Am. Water Resour. Assoc. 1986, 22, 471–478. [Google Scholar] [CrossRef]
- Gasparini, A.; Custodio, E.; Fontes, J.-C.; Jimenez, J.; Nuñez, J. Exemple d′etude geochimique et isotopique de circulations aquiferes en terrain volcanique sous climat semi–aride (Amurga, Gran Canaria, Iles Canaries). J. Hydrol. 1990, 114, 61–91. (In French) [Google Scholar] [CrossRef]
- Consejo Insular de Aguas de Gran Canaria. Hydrological Plan of Gran Canaria; Consejo Insular de Aguas de Gran Canaria: Gran Canaria, Spain, 1999. [Google Scholar]
- Robins, N. Groundwater quality in Scotland: Major ion chemistry of the key groundwater bodies. Sci. Total Environ. 2002, 294, 41–56. [Google Scholar] [CrossRef]
- Piper, A.M. A graphic procedure in the geochemical interpretation of water–analyses. Eos Trans. Am. Geophys. Union 1944, 25, 914–928. [Google Scholar] [CrossRef]
- Hutchins, M.; SmithM, B.; Rawlins, B.; Lister, T. Temporal and spatial variability of stream waters in Wales, the Welsh borders and part of the West Midlands, UK—1. Major ion concentrations. Water Res. 1999, 33, 3479–3491. [Google Scholar] [CrossRef]
- Custodio, E.; Alcalá-García, F.J. The potential of the Cl/Br ratio as indicator of the origin of the salinity of the Spanish coastal aquifers (El potencial de la relación Cl/Br como indicador del origen de la salinidad de los acuíferos costeros españoles). In Coastal Aquifers Intrusion Technology, Mediterranean Countries; IGME: Madrid, Spain, 2003; pp. 401–412. [Google Scholar]
- Ministry of the Spanish Presidency RD 140/2003. Technical—Sanitarian Regulation for the Supply and the Quality Control of the potable waters (Reglamentación técnico–sanitaria para el abastecimiento y el control de calidad de las aguas potables); (80/778/CEE); 2003. Available online: https://www.boe.es/buscar/pdf/2003/BOE-A-2003-3596-consolidado.pdf (accessed on 10 April 2019).










| Well | Latitude | Longitude | Elevation (m a.s.l.) |
|---|---|---|---|
| 1 | 27°50′52.04″ N | 15°29′00.20″ W | 160 |
| 2 | 27°47′51.71″ N | 15°40′08.14″ W | 85 |
| 3 | 27°48′14.66″ N | 15°40′06.16″ W | 85 |
| 4 | 27°48′44.62″ N | 15°39′54.10″ W | 85 |
| 5 | 27°49′40.05″ N | 15°39′42.20″ W | 115 |
| 6 | 27°49′58.37″ N | 15°29′39.63″ W | 140 |
| 7 | 27°48′38.86″ N | 15°28′39.77″ W | 40 |
| 8 | 27°50′06.72″ N | 15°28′37.08″ W | 125 |
| 9 | 27°49′06.21″ N | 15°27′28.87″ W | 60 |
| 10 | 27°48′21.88″ N | 15°28′17.50″ W | 25 |
| 11 | 27°49′37.22″ N | 15°27′45.85″ W | 90 |
| 12 | 27°49′11.25″ N | 15°27′56.59″ W | 65 |
| 13 | 27°49′42.04″ N | 15°27′12.67″ W | 65 |
| 14 | 27°50′29.97″ N | 15°28′02.16″ W | 115 |
| 15 | 27°50′40.38″ N | 15°27′59.46″ W | 120 |
| 16 | 27°49′15.14″ N | 15°26′50.37″ W | 45 |
| Parameter | Container 1 | Conservation of the Sample | Max. Time for Analysis | Norm |
|---|---|---|---|---|
| Conductivity | P, G | Refrigeration 2–5 °C | 28 days | (ISO 7888, 1985) |
| pH | P, G | Directly analysis | 2 h | (APHA–AWWA–WPCF, 1992i) |
| Bicarbonates | Refrigeration 2–5 °C | 14 days | (ISO 9963–1, 1996) | |
| Alkalinity (TAC) | P, G | Refrigeration 2–5 °C | 24 h | (ISO 9963–1, 1996) |
| Sulfates | P, G | Refrigeration 2–5 °C | 28 days | (APHA–AWWA–WPCF, 1992m) |
| Nitrates | P, G | Refrigeration 2–5 °C | 48 h | (APHA–AWWA–WPCF, 1992k) |
| Calcium | P, G | Refrigeration 2–5 °C | 6 months | (APHA–AWWA–WPCF, 1992d) |
| Magnesium | P, G | Refrigeration 2–5 °C | 24 h | (APHA–AWWA–WPCF, 1992c) |
| TDS | P, G | Refrigeration 2–5 °C | 2–7 days | (APHA–AWWA–WPCF, 1992c) |
| Chlorides | P, G | Refrigeration 2–5 °C | 24 h | (APHA–AWWA–WPCF, 1992h) |
| Sodium | P, G | Refrigeration 2–5 °C | 6 months | (APHA–AWWA–WPCF, 1992g) |
| Potassium | P, G | Refrigeration 2–5 °C | 6 months | (APHA–AWWA–WPCF, 1992f) |
| Silica | P | Refrigeration, no freeze | 28 days | (APHA–AWWA–WPCF, 1992l) |
| Type of Water | Na/Cl | (Na-Cl)/SO4 | (Cl-Na)/Mg |
|---|---|---|---|
| Chloride-calcium | <1 | <0 | >1 |
| Chloride-magnesium | <1 | <0 | <1 |
| Sodium-bicarbonate | >1 | >1 | <0 |
| Sodium-sulfate | >1 | <1 | <0 |
| Well ID n° | HCO3 | SO4 | Cl | NO3 | Ca | Mg | Na | K | SiO2 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 191 (92–305) | 191 (148–323) | 2519 (1715–3362) | 5.14 (1–9.6) | 529 (368–783) | 389 (264–483) | 483 (257–830) | 37.7 (17–85) | 48.7 (30–65) |
| 2 | 168 (91–336) | 96.9 (59–194) | 500 (325–600) | 4.17 (1.4–8.2) | 56.5 (37.5–79) | 41.3 (13.2–52) | 318 (195–595) | 19.5 (8.8–27) | 45.2 (14.8–54.2) |
| 3 | 157 (88–290) | 74.0 (42–122) | 445 (344–532) | 4.43 (1.5–8) | 65.0 (40–90) | 50.2 (41–81) | 214 (163–268) | 15.4 (12–22) | 45.5 (29.8–64.5) |
| 4 | 212 (107–365) | 70.9 (24–131) | 374 (328–450) | 3.89 (1.3–6.2) | 60.9 (53–78) | 41.0 (17–77) | 215 (152–263) | 13.6 (10–18) | 40.2 (20.4–47) |
| 5 | 96.2 (55–161) | 128 (58–208) | 662 (510–781) | 4.67 (1.7–8.05) | 81.4 (70–128) | 52.9 (26–106) | 330 (250–425) | 15.8 (11–21) | 31.2 (17–36) |
| 6 | 288 (150–368) | 40.2 (24–84) | 216 (170–270) | 3.84 (1.1–10.9) | 47.1 (28–66.9) | 40.0 (21–51) | 143 (97–175) | 11.0 (8.2–15) | 80.1 (47–94) |
| 7 | 247 (146–344) | 132 (77–217) | 555 (376–669) | 6.37 (0.16–12.8) | 79 (59–113) | 73.4 (43–167) | 289 (200–365) | 16.5 (10–22) | 39.5 (18.5–45) |
| 8 | 242 (66–479) | 82.7 (39–195) | 371 (240–683) | 8.41 (1.3–24.6) | 63.0 (45–112) | 50.5 (40–62) | 210 (148–355) | 13.1 (9.9–20) | 41.5 (23–64.4) |
| 9 | 229 (128–315) | 58.2 (28–124) | 251 (125–325) | 6.95 (1.3–13) | 46.2 (35–66) | 37.5 (29–45) | 161 (120–190) | 11.36 (8.2–15) | 39.1 (19.1–92) |
| 10 | 211 (91.5–280) | 176 (115–481) | 497 (205–975) | 21.3 (1–73.9) | 95.2 (59–128) | 64.9 (25–115) | 269 (172–449) | 18.4 (11.7–24) | 34.6 (25–67) |
| 11 | 195 (70–286) | 125 (70–341) | 304 (191–806) | 15.4 (1.3–47.7) | 27.8 (5–90.4) | 33.9 (14–72.8) | 246 (147–617) | 16.4 (9.4–23) | 27.9 (12.6–65) |
| 12 | 296 (128–480) | 169 (53–696) | 536 (161–1377) | 26.0 (1.2–189) | 49.2 (27–136) | 56.4 (24.8–152) | 365 (116–788) | 17.5 (9–25) | 25.5 (14.1–30) |
| 13 | 305 (174–416) | 446 (240–750) | 2,129 (1377–4200) | 42.4 (1.7–189) | 349 (136–1090) | 284 (148–709) | 774 (500–1150) | 33.5 (23–50) | 31.9 (13–48) |
| 14 | 233 (134–342) | 342 (42–932) | 2999 (459–4100) | 14.8 (0.3–167) | 581 (117–783) | 477 (73.7–821) | 662 (122–1040) | 39.1 (10–53) | 46.2 (14.2–54.3) |
| 15 | 212 (104–332) | 389 (249–760) | 3533 (368–4154) | 5.32 (0.4–17) | 644 (450–1109) | 601 (347–695) | 804 (408–1150) | 51.4 (11–92) | 44.9 (26.9–52) |
| 16 | 202 (107–562) | 573 (137–860) | 2851 (676–3830) | 43.0 (0.4–117) | 697 (171–1182) | 435 (116–665) | 572 (142–1660) | 41.1 (10–59) | 38 (16.2–62) |
| Well ID n° | CO3 | Fe | pH | Cond (µS/cm) | Salinity (mg/L) | TDS | SAR | Langelier-Hoover Index |
|---|---|---|---|---|---|---|---|---|
| 1 | 0.42 (0–9.3) | 0.32 (0.08–1.22) | 7.32 (6.94–8.18) | 7716 (5590–8770) | 4400 (3600–4800) | 4676 (2900–6766) | 3.69 (2.4–6.3) | 0.59 Incrust |
| 2 | 0.70 (0–9.2) | 0.22 (0.04–0.90) | 7.78 (7.5–8.4) | 2128 (152–2,640) | 1180 (941–1385) | 1183 (846–1498) | 7.45 (4.9–9.7) | 0.66 Incrust |
| 3 | 0.53 (0–6.5) | 0.12 (0.04–0.50) | 7.73 (7–8.02) | 1906 (1695–2230) | 945 (844–1138) | 1005 (780–415) | 4.62 (0.4–7.6) | 0.64 Incrust |
| 4 | 0.18 (0–4.3) | 0.21 (0.02–1.58) | 7.69 (7.48–8) | 1810 (1581–2729) | 913 (786–1437) | 946 (750–1163) | 5.25 (3.8–6.9) | 0.74 Incrust |
| 5 | 0.85 (0–9.4) | 1.04 (0.02–15.90) | 7.87 (7.45–8.05) | 2708 (2,462–3090) | 1436 (1277–1654) | 1330 (1055–1891) | 7.13 (5.3–10.8) | 0.68 Incrust |
| 6 | 0.96 (0–22) | 0.15 (0.04–0.57) | 7.27 (7–7.73) | 1314 (1,095–1581) | 645 (538–786) | 748 (550–872) | 3.75 (2.7–6) | 0.27 Incrust |
| 7 | 1.28 (0–30.8) | 0.18 (0.05–0.60) | 7.44 (7.13–7.7) | 2569 (1680–2950) | 1343 (837–1,570) | 1378 (100–1831) | 5.73 (3–8.1) | 0.56 Incrust |
| 8 | 0.48 (0–11.4) | 0.65 (0.03–11.40) | 7.89 (7.32–8.2) | 1750 (1450–2617) | 878 (719–1370) | 977 (800–1346) | 4.83 (3.4–7.8) | 0.44 Incrust |
| 9 | - | 0.18 (0.02–0.58) | 7.57 (7.17–8.22) | 1334 (800–1958) | 660 (389–979) | 757 (610–1010) | 4.11 (2–5.6) | 0.56 Incrust |
| 10 | 0.51 (0–12.2) | 1.69 (0.05–15.70) | 7.75 (7.42–8) | 2339 (1300–3870) | 1167 (643–1,589) | 1322 (974–2256) | 5.28 (3.75–6.7) | 0.98 Incrust |
| 11 | 20.4 (0–100) | 4.41 (0.07–93) | 8.80 (7.76–9.85) | 1659 (900–3810) | 724 (535–913) | 900 (530–2204) | 7.71 (3.6–15.3) | 1.65 Incrust |
| 12 | 2.95 (0–19) | 0.16 (0.03–0.70) | 7.96 (7.63–8.23) | 2409 (1151–5760) | 1098 (567–1330) | 1356 (510–3548) | 8.92 (3.8–12.8) | 1.03 Incrust |
| 13 | 0.89 (0–21.4) | 0.19 (0.03–0.69) | 7.51 (7.1–8) | 6599 (2500–8750) | 3630 (1300–4775) | 4357 (3,100–8845) | 8.58 (3.8–14.2) | 0.78 Incrust |
| 14 | - | 0.56 (0.05–6.90) | 7.25 (6.9–7.91) | 9680 (1715–11,620) | 5735 (4720–6391) | 5486 (1182–8384) | 5.11 (2.49–15) | 0.66 Incrust |
| 15 | - | 0.27 (0.04–1.32) | 7.19 (7–7.78) | 11762 (7250–13,410) | 7868 (5736–8646) | 6732 (3904–10,299) | 5.50 (3–8.3) | 0.64 Incrust |
| 16 | - | 0.26 (0.05–1.90) | 7.2 (6.9–8.04) | 9862 (1917–13,350) | 5810 (958–8610) | 5867 (514–11,135) | 4.44 (2.3–13.7) | 0.55 Incrust |
| Parameter | Unit | Maximum Level |
|---|---|---|
| Calcium | mg/L Ca | - |
| Chloride | mg/L Cl– | 250 |
| Sulfate | mg/L SO4 | 250 |
| Nitrite | mg/L NO2– | 0.1 |
| Nitrate | mg/L NO3– | 50 |
| pH | - | 6.5–9.5 |
| Potassium | mg/L K | 12 |
| Magnesium | mg/L Mg | 50 |
| Sodium | mg/L Na | 150 |
| Conductivity | µS/cm | 2500 |
| TDS | mg/L | 1500 |
| Langelier-Hoover index | - | ±0.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-García, A.; Carrascosa-Chisvert, M.D.; Mena, V.; Souto, R.M.; Santana, J.J.; Nuez, I. Groundwater Quality Assessment in a Volcanic Mountain Range (South of Gran Canaria Island, Spain). Water 2019, 11, 754. https://doi.org/10.3390/w11040754
Ruiz-García A, Carrascosa-Chisvert MD, Mena V, Souto RM, Santana JJ, Nuez I. Groundwater Quality Assessment in a Volcanic Mountain Range (South of Gran Canaria Island, Spain). Water. 2019; 11(4):754. https://doi.org/10.3390/w11040754
Chicago/Turabian StyleRuiz-García, A., M.D. Carrascosa-Chisvert, V. Mena, R.M. Souto, J.J. Santana, and I. Nuez. 2019. "Groundwater Quality Assessment in a Volcanic Mountain Range (South of Gran Canaria Island, Spain)" Water 11, no. 4: 754. https://doi.org/10.3390/w11040754